Fates of Pyruvate After Glycolysis: A Metabolic Crossroads
Explore the multiple fates of pyruvate after glycolysis — from lactate and acetyl-CoA formation to gluconeogenesis and fermentation. A detailed guide for postgraduate biotechnology and microbiology students based on LibreTexts and current biochemical insights.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
10/8/20257 min read

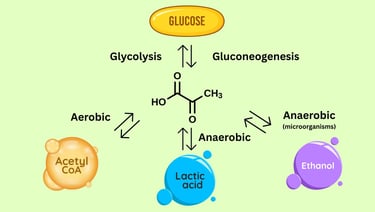
After glycolysis, every cell faces a critical biochemical decision — what to do with the two molecules of pyruvate produced from one glucose molecule. For postgraduate students in biotechnology and microbiology, understanding the diverse fates of pyruvate is central to grasping how cells balance energy production, biosynthesis, and redox homeostasis.
Pyruvate acts as a metabolic hub, linking carbohydrate metabolism with lipid, amino acid, and energy pathways. Its subsequent transformation depends on several factors: oxygen availability, the cell’s energy state, and its metabolic demands.
1. Pyruvate to Lactate: Anaerobic Adaptation
When oxygen supply is limited or cellular demand for ATP exceeds the capacity of oxidative phosphorylation, cells switch to anaerobic metabolism. In this state, pyruvate—the end product of glycolysis—undergoes reduction to lactate, catalyzed by the enzyme lactate dehydrogenase (LDH).
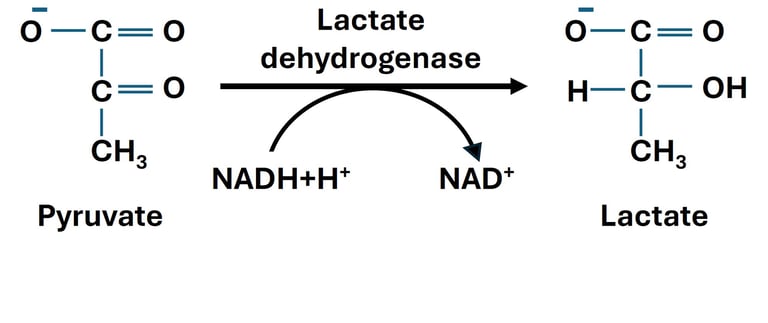
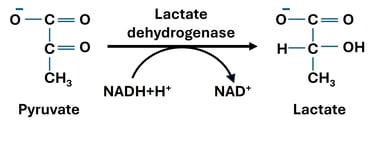
This reaction is reversible and plays a crucial role in maintaining the redox balance within the cytoplasm. During glycolysis, NAD⁺ is reduced to NADH when glyceraldehyde-3-phosphate is oxidized. If oxygen is unavailable, NADH cannot donate its electrons to the mitochondrial electron transport chain. The conversion of pyruvate to lactate thus regenerates NAD⁺, allowing glycolysis to continue producing ATP through substrate-level phosphorylation.
Physiological Significance
Muscle cells: During intense exercise, oxygen delivery to muscles may not meet the energy demand. Lactate accumulation enables continued ATP production but also lowers intracellular pH, contributing to fatigue.
Red blood cells: These cells lack mitochondria entirely, relying solely on glycolysis and LDH-mediated lactate formation for ATP.
Certain microorganisms: Many bacteria and protozoa also reduce pyruvate to lactate during lactic acid fermentation, an energy-efficient survival mechanism in anaerobic habitats.
2. Pyruvate to Acetyl-CoA: The Gateway to the TCA Cycle
When oxygen is available, pyruvate enters the mitochondrial matrix through a transporter and undergoes oxidative decarboxylation catalyzed by the pyruvate dehydrogenase complex (PDC).
Reaction:
Pyruvate + CoA + NAD⁺ → Acetyl-CoA + CO₂ + NADH + H⁺
This irreversible step connects glycolysis to the tricarboxylic acid (TCA) cycle. The generated acetyl-CoA is further oxidized to yield CO₂, NADH, FADH₂, and ATP through oxidative phosphorylation.
PDC is tightly regulated:
Inhibited by: NADH, Acetyl-CoA, ATP
Activated by: ADP, NAD⁺, CoA
Regulated via phosphorylation by pyruvate dehydrogenase kinase (PDK)
This control ensures energy production matches cellular demand.
3. Pyruvate to Oxaloacetate: Anaplerosis and Gluconeogenesis
In cells needing biosynthetic intermediates, pyruvate is carboxylated to oxaloacetate (OAA) by pyruvate carboxylase, a biotin-dependent enzyme found in mitochondria.
Reaction:
Pyruvate + CO₂ + ATP → Oxaloacetate + ADP + Pi
OAA replenishes TCA intermediates (anaplerotic function) or serves as a precursor for gluconeogenesis in liver and kidney. In gluconeogenic tissues, OAA is converted to phosphoenolpyruvate (PEP) by PEP carboxykinase and subsequently to glucose.
This reaction is a crucial link between energy metabolism and biosynthetic processes.
4. Pyruvate to Alanine: Linking Carbon and Nitrogen Metabolism
Through transamination, pyruvate can accept an amino group from glutamate to form alanine, catalyzed by alanine aminotransferase (ALT):
Reaction:
Pyruvate + Glutamate ↔ Alanine + α-Ketoglutarate
This reversible process forms part of the alanine cycle, allowing muscle tissues to transfer amino groups and carbon skeletons to the liver. The liver converts alanine back to pyruvate for gluconeogenesis, while disposing of nitrogen via urea synthesis.
It highlights pyruvate’s central role in integrating carbon and nitrogen metabolism.
5. Pyruvate to Malate and NADPH Generation
In some tissues and microorganisms, pyruvate participates in the malic enzyme route, where malic enzyme catalyzes the reversible conversion between pyruvate and malate:
Reaction:
Malate + NADP⁺ ↔ Pyruvate + CO₂ + NADPH
This reaction supports lipid biosynthesis and redox balance, as NADPH is essential for anabolic processes. Pyruvate–malate cycling also helps shuttle reducing equivalents between mitochondria and cytosol.
6. Alcoholic Fermentation (Ethanolic Fermentation)
Prominent in yeasts (e.g., Saccharomyces cerevisiae) and some bacteria, this pathway converts pyruvate to ethanol and carbon dioxide.
Stepwise reactions:
Pyruvate decarboxylase (PDC):
CH₃COCOOH → CH₃CHO + CO₂Alcohol dehydrogenase (ADH):
CH₃CHO + NADH + H⁺ → CH₃CH₂OH + NAD⁺
Net reaction:
C₆H₁₂O₆ → 2 C₂H₅OH + 2 CO₂
This process allows continuous NAD⁺ regeneration, sustaining glycolytic ATP production.
Industrial relevance: Brewing, winemaking, bioethanol production.
7. Summary Table
FAQ
1. Why is pyruvate considered a central metabolic intermediate?
Pyruvate is the end product of glycolysis and serves as a key junction connecting several metabolic pathways. It can be oxidized to acetyl-CoA for the TCA cycle, reduced to lactate or ethanol during fermentation, converted to oxaloacetate for gluconeogenesis, or used in amino acid and fatty acid biosynthesis. This versatility makes pyruvate a metabolic crossroads between energy generation and biosynthesis.
2. What determines the fate of pyruvate in a cell?
The fate depends primarily on the availability of oxygen, cell type, and organism:
In aerobic conditions, pyruvate is oxidatively decarboxylated to acetyl-CoA and enters the TCA cycle.
In anaerobic conditions, it undergoes fermentation to regenerate NAD⁺, producing lactate, ethanol, or other organic acids.
In microbes, environmental factors like pH, substrate availability, and redox potential further influence which fermentation pathway is dominant.
3. How does pyruvate conversion to lactate differ in humans and microbes?
In humans and other animals, lactate dehydrogenase (LDH) converts pyruvate to lactate during oxygen deficiency (anaerobic glycolysis), such as in muscle tissues.
In microorganisms, lactate formation is part of lactic acid fermentation, which can be either homolactic (producing mainly lactate) or heterolactic (producing lactate along with ethanol, CO₂, and acetate).
4. What enzymes are primarily responsible for pyruvate metabolism?
Key enzymes include:
Pyruvate dehydrogenase complex (PDH): Converts pyruvate to acetyl-CoA.
Lactate dehydrogenase (LDH): Reduces pyruvate to lactate.
Pyruvate decarboxylase (PDC): Decarboxylates pyruvate to acetaldehyde (ethanol fermentation).
Pyruvate formate-lyase (PFL): Converts pyruvate to formate and acetyl-CoA in anaerobes.
Pyruvate:ferredoxin oxidoreductase (PFOR): Generates acetyl-CoA and CO₂ in strict anaerobes.
5. What is the significance of pyruvate fermentation in biotechnology?
Microbial pyruvate fermentation underpins numerous industrial processes, such as:
Lactic acid production (food preservation, biodegradable plastics).
Ethanol fermentation (biofuels, beverages).
Butanol and acetone production (solvent industry).
Succinate and propionate formation (organic acid and vitamin production).
It’s also used in anaerobic digestion and biohydrogen generation for sustainable energy.
6. How is pyruvate metabolism linked to the TCA cycle?
Under aerobic conditions, pyruvate is converted to acetyl-CoA by the pyruvate dehydrogenase complex, which then enters the TCA cycle for complete oxidation to CO₂ and H₂O. This process yields NADH and FADH₂, which drive ATP production through oxidative phosphorylation.
7. Can pyruvate be used for biosynthesis?
Yes. Pyruvate is a precursor for several anabolic pathways, including:
Amino acid synthesis (alanine via transamination).
Gluconeogenesis (via oxaloacetate).
Fatty acid synthesis (through acetyl-CoA).
Thus, pyruvate connects energy metabolism with macromolecular biosynthesis.
8. Why do microbes have multiple pyruvate fermentation pathways?
Microbes have evolved diverse fermentative routes to adapt to fluctuating oxygen, nutrient, and redox conditions. This metabolic flexibility allows them to survive in various ecological niches — from the human gut to anaerobic sediments — while optimizing energy yield and maintaining redox balance.
9. How is pyruvate metabolism studied in modern biotechnology?
Advanced techniques such as metabolic flux analysis (MFA), isotopic labeling (¹³C-NMR, GC-MS), and metabolic engineering are used to analyze and optimize pyruvate pathways. These approaches help enhance yields in fermentation-based industries and develop engineered microbes for biofuel and bioproduct synthesis.
10. What happens to pyruvate in oxygen-limited tissues like tumors?
In rapidly proliferating or hypoxic tissues (such as tumor cells), pyruvate is preferentially converted to lactate even when oxygen is available — a phenomenon known as the Warburg effect. This allows continuous ATP generation via glycolysis and provides intermediates for biosynthetic pathways essential for cell proliferation.
Critical Practice Questions: Fates of Pyruvate After Glycolysis
A. Conceptual and Short-Answer Questions
Why is pyruvate considered a metabolic hub?
Discuss how its conversion pathways link catabolism and anabolism in cellular metabolism.Explain how redox balance influences the fate of pyruvate in different organisms.
Compare the enzymatic steps of alcoholic fermentation and lactic acid fermentation.
Include coenzyme requirements and energy yield.What are the roles of pyruvate dehydrogenase (PDH) and pyruvate formate-lyase (PFL)?
How do their mechanisms reflect adaptation to aerobic and anaerobic conditions?Describe how fermentation pathways maintain ATP production in the absence of oxygen.
In what ways is pyruvate metabolism regulated by cellular energy charge (ATP/ADP/AMP ratio)?
Outline the conversion of pyruvate to oxaloacetate and discuss its role in gluconeogenesis.
What is the biochemical significance of the Warburg effect in cancer cells in relation to pyruvate metabolism?
Differentiate between homolactic and heterolactic fermentation in terms of enzymes, products, and microorganisms.
Discuss the role of pyruvate:ferredoxin oxidoreductase (PFOR) in strict anaerobes.
How does it differ from PDH in reaction mechanism and electron acceptors?
B. Analytical and Application-Based Questions
A yeast culture is shifted from aerobic to anaerobic conditions.
Predict how pyruvate metabolism will change, including key enzymes and end products.Given that glycolysis produces 2 NADH per glucose molecule, explain how fermentation pathways regenerate NAD⁺ to sustain glycolysis.
In mixed acid fermentation, how does E. coli decide which end products to produce under varying pH and redox conditions?
Design an experiment to determine whether a microorganism performs lactic acid or alcoholic fermentation.
Describe the culture conditions, indicators, and analytical methods.Why might engineered microbes be designed to divert pyruvate toward succinate or lactate production?
Discuss the metabolic and industrial rationale.Explain how the fate of pyruvate differs between animal muscle cells under hypoxia and Clostridium species in anaerobic fermentation.
Compare energy yields (ATP per glucose molecule) among glycolysis coupled with:
Aerobic respiration
Lactic acid fermentation
Ethanol fermentation
How can isotopic labeling of carbon in pyruvate help track its metabolic fate in cellular systems?
Evaluate how pyruvate metabolism connects with amino acid biosynthesis, particularly for alanine, aspartate, and glutamate.
In industrial bioreactors, how does controlling oxygen tension and pH influence the distribution of pyruvate-derived fermentation products?
C. Case Study / Research-Oriented Questions
Case Study:
A Clostridium acetobutylicum strain shows reduced butanol yield. Suggest possible enzymatic or regulatory bottlenecks in its pyruvate metabolism.Discuss how metabolic engineering could be applied to redirect pyruvate flux toward bioethanol or lactic acid production in microorganisms.
Propose how CRISPR-based knockout of lactate dehydrogenase might alter the metabolic fate of pyruvate in E. coli.
Describe how pyruvate metabolism is integrated into global carbon flux in anaerobic ecosystems, such as sediments or the gut microbiome.
Critically analyze the role of pyruvate in linking glycolysis, the TCA cycle, and fermentation in facultative anaerobes.
Further Readings
Glycolysis: Steps, Functions, and the Universal Energy Pathway : https://biotechbeacon.com/glycolysis-steps-functions-and-the-universal-energy-pathway
Basics of Metabolism: Definition, Types, Pathways & Importance in Biology : https://biotechbeacon.com/basics-of-metabolism-definition-types-pathways-and-importance-in-biology
Glucose Transporters (GLUTs): Gatekeepers of Cellular Energy : https://biotechbeacon.com/glucose-transporters-gluts-gatekeepers-of-cellular-energy
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.