Glycolysis: Steps, Functions, and the Universal Energy Pathway
Understand glycolysis: pathway steps, regulation, ATP production, NADH generation, and its importance in cellular respiration and health.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
9/7/20258 min read
The role of glycolysis in metabolism
The glycolytic pathway is a conserved metabolic pathway that facilitates the anaerobic breakdown of glucose while simultaneously generating ATP. The pathway comprises 10 metabolic enzymes that function sequentially to transform a six-carbon glucose molecule into two three-carbon molecules of the tricarboxylic acid cycle precursor, pyruvate. The anaerobic conversion transpires in the cytoplasm, yielding a net production of two ATP molecules, two reduced nicotinamide adenine dinucleotide (NADH) molecules, and two water molecules for each glucose molecule that enters the route. In the presence of physiological oxygen (O2) levels, pyruvate produced from glycolysis is carried into the mitochondria, where it is oxidized in an O2-dependent manner to yield acetyl coenzyme A (acetyl co-A). Acetyl CoA is processed via the tricarboxylic acid cycle and the electron transport chain during oxidative phosphorylation, yielding a substantially higher quantity of ATP per glucose molecule compared to glycolysis alone.
Principals of Glycolysis
Glycolysis happens in both aerobic and anaerobic conditions. Under aerobic conditions, pyruvate enters the citric acid cycle and undergoes oxidative phosphorylation, resulting in the net generation of 32 ATP molecules. Under anaerobic circumstances, pyruvate is converted to lactate by anaerobic glycolysis. Anaerobic respiration yields 2 ATP molecules. Glucose is a hexose sugar, indicating it is a monosaccharide of six carbon atoms and six oxygen atoms. The initial carbon possesses an attached aldehyde group, whereas the other five carbons each contain a hydroxyl group. In glycolysis, glucose is metabolized into pyruvate and energy, yielding a total of 2 ATP (Glucose + 2 NAD+ + 2 ADP + 2 Pi --> 2 Pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O). The hydroxyl groups facilitate phosphorylation. The particular kind of glucose utilized in glycolysis is glucose 6-phosphate.
Cellular Level of Glycolysis
Glycolysis takes place in the cytoplasm of cells. In aerobic conditions, pyruvate obtained from glucose will reach the mitochondria to perform oxidative phosphorylation. Anaerobic conditions cause pyruvate to remain in the cytoplasm, where it is converted to lactate by the enzyme lactate dehydrogenase.
Steps at the Molecular Level
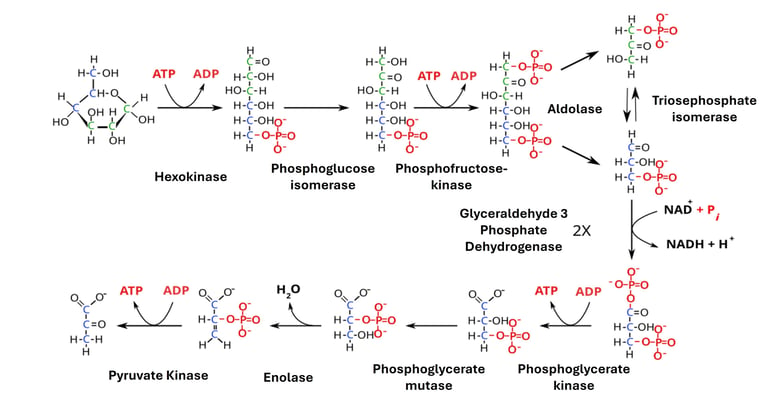
The glycolytic pathway involves a sequence of enzyme-catalyzed reactions:
Glucose → Glucose-6-phosphate (via hexokinase or glucokinase, using ATP).
Glucose-6-phosphate → Fructose-6-phosphate (isomerization step).
Fructose-6-phosphate → Fructose-1,6-bisphosphate (using another ATP, catalyzed by phosphofructokinase, the key regulatory enzyme).
Fructose-1,6-bisphosphate → DHAP + G3P (aldolase splits the sugar).
DHAP ↔ G3P (triosephosphate isomerase converts DHAP into G3P).
G3P → 1,3-bisphosphoglycerate (NAD+ reduced to NADH).
1,3-bisphosphoglycerate → 3-phosphoglycerate (first ATP formed).
3-phosphoglycerate → 2-phosphoglycerate (via mutase).
2-phosphoglycerate → Phosphoenolpyruvate (PEP) (via enolase).
PEP → Pyruvate (pyruvate kinase produces the second ATP).
Net result:
2 ATP (after accounting for 2 used, 4 produced)
2 NADH
2 Pyruvate
Function and Significance
Energy production: Glycolysis provides ATP rapidly, even when oxygen is limited.
Versatility: Pyruvate serves as a precursor for many biosynthetic pathways, including amino acids and fatty acids.
Backup system: In oxygen-rich cells, glycolysis prepares substrates for oxidative phosphorylation; in oxygen-poor environments, it ensures survival through fermentation.
Special tissues:
Red blood cells rely entirely on glycolysis since they lack mitochondria.
The eye lens depends on glycolysis to avoid light-scattering mitochondria.
Muscle cells use both glycolysis and aerobic respiration, especially during intense exercise.
Glucose transporter
The amount of glucose available for the process regulates glycolysis, which becomes available primarily in two ways:
Regulation of glucose reuptake or regulation of the breakdown of glycogen.
Glucose transporters (GLUT) transport glucose from the outside of the cell to the inside. Cells containing GLUT can increase the number of GLUT in the cell's plasma membrane from the intracellular matrix, therefore increasing the uptake of glucose and the supply of glucose available for glycolysis.
There are five types of GLUTs. GLUT1 is present in RBCs, the blood-brain barrier, and the blood-placental barrier.
GLUT2 is in the liver, beta-cells of the pancreas, kidney, and gastrointestinal (GI) tract.
GLUT3 is present in neurons.
GLUT4 is in adipocytes, heart, and skeletal muscle.
GLUT5 specifically transports fructose into cells.
Another form of regulation is the breakdown of glycogen. Cells can store extra glucose as glycogen when glucose levels are high in the cell plasma. Conversely, when levels are low, glycogen can be converted back into glucose.
Two enzymes control the breakdown of glycogen: glycogen phosphorylase and glycogen synthase. The enzymes can be regulated through feedback loops of glucose or glucose 1-phosphate, or via allosteric regulation by metabolites, or from phosphorylation/dephosphorylation control.
Oxygen and Allosteric Regulators
Numerous enzymes participate in the glycolytic route by changing one intermediate into another, as previously mentioned. Glycolysis can be controlled by regulating these enzymes, which include pyruvate kinase, phosphofructokinase, glyceraldehyde-3-phosphate dehydrogenase, and hexokinase. Glycolysis can also be controlled by the amount of accessible oxygen. The "Pasteur effect" explains how the effect of glycolysis is lessened by oxygen availability, and how, at least initially, decreasing availability causes glycolysis to accelerate. Hexokinase and other allosteric regulators of glycolysis are among the processes causing this impact. Myocytes and hepatocytes are two examples of tissue with high mitochondrial capabilities where the "Pasteur effect" seems to be most prevalent. However, not all oxidative tissue, including pancreatic cells, will experience this effect.
Enzyme Induction and Regulation
A further strategy for regulating glycolytic processes is the transcriptional regulation of glycolytic enzymes. Modifying the level of essential enzymes enables the cell to adjust and adapt to changes in hormonal conditions. Elevated glucose and insulin levels can enhance the activity of hexokinase and pyruvate kinase, hence augmenting pyruvate synthesis.
PFK-1 in Glycolytic Regulation
Fructose 2,6-bisphosphate is an allosteric modulator of PFK-1. When there is a lot of fructose 2,6-bisphosphate, PFK-1 works more. Phosphofructokinase-2 (PFK-2) is responsible for making it. PFK-2 can serve as both a kinase and a phosphorylase. It can change fructose 6 phosphates into fructose 2,6-bisphosphate and back again. Insulin removes a phosphate group from PFK-2, which turns on its kinase activity. This makes fructose 2,6-bisphosphate, which then turns on PFK-1. Glucagon can also add a phosphate group to PFK-2, which turns on phosphatase and changes fructose 2,6-bisphosphate back into fructose 6-phosphate. This process lowers the amounts of fructose 2,6-bisphosphate and slows down the activity of PFK-1.
Mechanism of Glycolysis
There are two parts to glycolysis: the investment phase and the payoff phase. The investment phase is when energy, in the form of ATP, is added. The reward phase is when more ATP and NADH molecules are made than are used. In the investment phase, 2 ATP goes in, and in the reward phase, 4 ATP comes out. This means that there is a net total of 2 ATP. Substrate-level phosphorylation is the designation for the process by which fresh ATP is made.
Investment Stage
This step adds two phosphates to glucose. Hexokinase phosphorylates glucose into glucose-6 phosphate (G6P) to start glycolysis. This phase is where the first phosphate group is moved and the first ATP is used up. This is also a irreversible step that can't be undone. This phosphorylation keeps the glucose molecule inside the cell because it can't easily get through the cell membrane. Phosphoglucose isomerase changes G6P into fructose 6-phosphate (F6P) from there. Then, the second phosphate is added by phosphofructokinase (PFK-1). The second ATP is used by PFK-1 to turn F6P into fructose 1,6-bisphosphate. This step is also irreversible and is the one that is also rate limiting in nature. In the next stage, fructose 1,6-bisphosphate is split into two molecules, which are then used by fructose-bisphosphate aldolase to make dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Triosephosphate isomerase changes DHAP into G3P. DHAP and G3p are in a state of balance, which means they change from one to the other.
The Payoff Phase
At the start of this phase, it's important to remember that there are two 3-carbon sugars for every glucose. The enzyme glyceraldehyde-3-phosphate dehydrogenase changes G3P into 1,3-diphosphoglycerate by changing NAD+ into NADH. Then, phosphoglycerate kinase takes away a phosphate group from 1,3-diphosphoglycerate to generate 3-phosphoglycerate and an ATP by substrate-level phosphorylation. At this time, one 3-carbon molecule makes one ATP while the other makes another ATP. Phosphoglycerate mutase changes 3-phosphoglycerate into 2-phosphoglycerate. Then, enolase changes 2-phosphoglycerate into phosphoenolpyruvate (PEP). In the last stage, pyruvate kinase changes PEP into pyruvate and phosphorylates ADP into ATP by substrate-level phosphorylation, making two additional ATP. This is also an irreversible step and can't be undone either. For every glucose molecule that goes in, 2 ATP come out, along with 4 ATP, 2 NADH, and 2 pyruvate molecules.
For glycolysis to keep going, NADH must go back to NAD+ in cells. If there is no NAD+, the payout phase will stop, which will cause glycolysis to back up. Oxidative phosphorylation is how NADH turns back into NAD+ in aerobic cells. Fermentation is how it happens in cells that don't need oxygen. Lactic acid fermentation and alcohol fermentation are the two main types of fermentation.
FAQs on Glycolysis
Q1. What is glycolysis?
Glycolysis is a ten-step metabolic pathway in which glucose is broken down into two molecules of pyruvate, generating ATP and NADH in the process.
Q2. Where does glycolysis occur in the cell?
It occurs in the cytoplasm of both prokaryotic and eukaryotic cells.
Q3. Is glycolysis aerobic or anaerobic?
Glycolysis itself is anaerobic; it does not require oxygen. However, the fate of pyruvate depends on oxygen availability.
Q4. What are the main products of glycolysis?
Two molecules of pyruvate, two NADH, and a net gain of two ATP molecules per glucose.
Q5. Why is glycolysis important?
It is the universal pathway for energy production in nearly all organisms and provides intermediates for other metabolic pathways.
Q6. What happens to pyruvate after glycolysis?
Under aerobic conditions: it enters the mitochondria and is converted into acetyl-CoA for the citric acid cycle.
Under anaerobic conditions: it is converted into lactate (in animals) or ethanol + CO₂ (in yeast).
Q7. Which enzymes regulate glycolysis?
The three key regulatory enzymes are hexokinase (or glucokinase in liver), phosphofructokinase-1 (PFK-1), and pyruvate kinase.
Q8. How much energy is invested and gained in glycolysis?
Two ATP molecules are invested in the preparatory phase, and four ATP molecules are produced in the payoff phase, resulting in a net gain of two ATP.
Q9. What role does NAD⁺ play in glycolysis?
NAD⁺ acts as an electron acceptor and is reduced to NADH during glycolysis, which can later be used in oxidative phosphorylation.
Q10. Does glycolysis occur in all living organisms?
Yes, glycolysis is a nearly universal pathway, present in bacteria, plants, fungi, and animals.
Q11. Can glycolysis function without mitochondria?
Yes. Since it occurs in the cytoplasm, glycolysis can take place even in cells without mitochondria, such as red blood cells.
Q12. What is substrate-level phosphorylation in glycolysis?
It refers to the direct generation of ATP from ADP using high-energy intermediates (1,3-bisphosphoglycerate and phosphoenolpyruvate).
Q13. How is glycolysis linked to diseases?
Abnormal glycolysis rates are linked to cancer (Warburg effect), diabetes, and metabolic disorders.
Q14. What is the Warburg effect?
It is the observation that cancer cells prefer glycolysis for ATP production, even in the presence of oxygen, leading to lactate accumulation.
Q15. Can glycolysis be inhibited?
Yes. Certain inhibitors like fluoride (inhibits enolase) and iodoacetate (inhibits glyceraldehyde-3-phosphate dehydrogenase) can block the pathway.
Critical Thinking Questions on Glycolysis
Why is glycolysis considered the “universal energy pathway”?
(Think about its presence in both aerobic and anaerobic organisms, and why evolution preserved it.)If oxygen is absent, why does glycolysis still continue, and how is NAD⁺ regenerated?
(Hint: fermentation pathways.)Why is phosphofructokinase-1 (PFK-1) called the “committed step” of glycolysis?
(Analyze its irreversibility and regulatory role.Why does red blood cell metabolism rely exclusively on glycolysis for ATP production?
(Consider the absence of mitochondria.)How would inhibiting pyruvate kinase affect the ATP yield of glycolysis?
(Trace the reactions and energy balance.)Why is the net ATP yield of glycolysis only two, even though four ATP are produced?
(Think about energy investment in the preparatory phase.)What would happen if NADH produced in glycolysis could not be oxidized back to NAD⁺?
(Impact on pathway continuity.)Why does glycolysis produce lactate in muscle cells during intense exercise?
(Explain the role of oxygen supply vs. energy demand.)How can glycolysis intermediates serve as precursors for biosynthetic pathways?
(Consider amino acid, lipid, and nucleotide synthesis.)Why do cancer cells upregulate glycolysis even in oxygen-rich conditions (Warburg effect)?
(Discuss rapid ATP needs and biosynthetic precursors.)
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.