Glucose Transporters (GLUTs): Gatekeepers of Cellular Energy
Learn about glucose transporters, including SGLTs and GLUTs, their structure, function, and role in glucose absorption and reabsorption in the body. A clear and detailed guide for students and researchers.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
9/11/20256 min read
Introduction
Glucose serves as a vital energy source for most living organisms. However, due to its size and polarity, glucose cannot easily cross the lipid bilayer of the cell membrane through simple diffusion. To facilitate its entry into cells, a diverse family of specialized transport proteins known as glucose transporters is required. These transporters are broadly categorized into two main groups: Sodium–Glucose Linked Transporters (SGLTs) and Facilitated Diffusion Glucose Transporters (GLUTs).
For detailed understanding of glycolysis read: Glycolysis: Steps, Functions, and the Universal Energy Pathway
Structure of SGLTs and GLUTs
The first member of the SGLT family to be discovered and extensively studied was SGLT1 (Sodium–Glucose Linked Transporter 1). This protein contains 14 transmembrane helices, with both its amino (NH2) and carboxyl (COOH) termini oriented toward the extracellular side. Members of the SGLT family are relatively large, with a molecular weight between 60–80 kDa and consisting of approximately 580–718 amino acids.
On the other hand, GLUT proteins consist of 12 transmembrane domains with their amino and carboxyl termini located intracellularly. Comparative sequence analyses reveal that GLUT isoforms share 28–65% amino acid similarity with GLUT1, which was the first identified facilitative glucose transporter. Based on structural and functional similarities, GLUTs are classified into three subfamilies: Class I, Class II, and Class III.
Physiology and Function of SGLTs and GLUTs
SGLTs transport glucose into cells via secondary active transport, working in symport with sodium ions. These transporters do not use ATP directly; instead, they rely on the sodium concentration gradient maintained by the sodium–potassium ATPase pump as a source of energy to move glucose against its concentration gradient.
In the human body, SGLTs are primarily located on the luminal surface of intestinal epithelial cells, where they mediate glucose absorption from digested food. They are also present in the renal proximal tubules, where they play a crucial role in reabsorbing glucose from the glomerular filtrate and preventing its loss in urine.
GLUTs: Facilitative Glucose Transporters
Facilitative glucose transporters (GLUTs) enable glucose to cross the plasma membrane through facilitated diffusion, a process that does not require energy. These transporters are widely distributed in tissues and play a critical role in maintaining glucose homeostasis.
Class I Facilitative Glucose Transporters
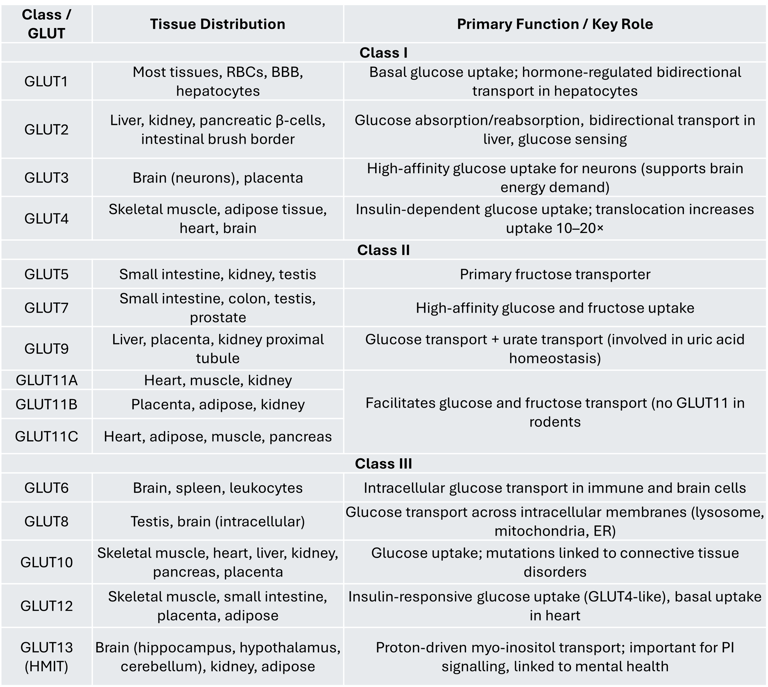
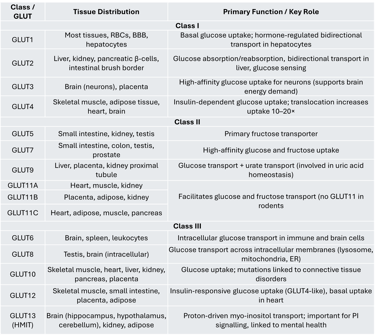
Class I transporters include GLUT1 through GLUT4, which are the most studied members of the GLUT family.
GLUT1 is expressed in many tissues and is particularly important for the hormone-regulated, bidirectional transport of glucose in hepatocytes. It is also influenced by thyroid hormones.
GLUT2 is primarily found in the liver, kidney, pancreatic β-cells, and intestinal brush border cells. In the intestine and kidney, it facilitates glucose absorption and reabsorption, respectively. In hepatocytes, GLUT2 regulates both glucose uptake and release, playing a key role in hepatic glucose metabolism. Interestingly, while β-cells of marine organisms use GLUT2 as a glucose sensor, human β-cells predominantly express GLUT1. Glucose sensing in pancreatic β-cells involves two steps: (1) glucose entry via GLUT transporters and (2) phosphorylation of glucose by glucokinase, which is considered the main glucose sensor according to animal and clinical studies.
GLUT3 is the principal glucose transporter in the brain. Its high affinity for glucose ensures efficient uptake even when blood glucose levels are low, making it ideal for neurons with high energy demands.
GLUT4 is an insulin-responsive transporter found in skeletal muscle, adipose tissue, heart, and brain. Under basal conditions, it resides in intracellular vesicles. In response to insulin, GLUT4 is translocated to the plasma membrane, resulting in a 10–20-fold increase in glucose uptake.
Class II Facilitative Glucose Transporters
Class II GLUTs include GLUT5, GLUT7, GLUT9, and GLUT11.
GLUT5 is the primary fructose transporter and is expressed in the small intestine, kidney, and testis. It plays crucial roles in both normal physiology and certain pathological conditions.
GLUT7 is present in the small intestine, colon, testis, and prostate, and has a high affinity for both glucose and fructose.
GLUT9 exists in multiple isoforms and is predominantly found in the liver, placenta, and kidney proximal tubules. It is also involved in urate transport, which has clinical relevance in gout.
GLUT11 shares about 42% sequence similarity with GLUT5 and is expressed in three isoforms:
GLUT11A: Found in the heart, skeletal muscle, and kidney
GLUT11B: Expressed in placenta, adipose tissue, and kidney
GLUT11C: Present in heart, skeletal muscle, adipose tissue, and pancreas
Unlike GLUT5, GLUT11 facilitates the transport of both glucose and fructose. Notably, rodents do not have a GLUT11 gene.
Class III Facilitative Glucose Transporters
Class III transporters include GLUT6, GLUT8, GLUT10, GLUT12, and GLUT13 (HMIT). Unlike Class I and II GLUTs, Class III members have their glycosylation site on loop 9 rather than loop 1.
GLUT6 is mainly expressed in the brain, spleen, and leukocytes.
GLUT8 is an intracellular, low-affinity glucose transporter found in the testis and brain. It does not undergo insulin-dependent translocation to the plasma membrane. GLUT8 facilitates glucose movement across intracellular organelles such as lysosomes, mitochondria, and the endoplasmic reticulum. Animal studies (Slc2a8 knockout mice) show that the absence of GLUT8 leads to mild changes in brain, heart, and sperm function, including reduced sperm motility and ATP levels.
GLUT10 is expressed in skeletal muscle, heart, lung, brain, liver, kidney, placenta, and pancreas.
GLUT12 is found in adipose tissue, small intestine, skeletal muscle, and placenta. Functionally similar to GLUT4, it translocates to the skeletal muscle cell membrane in response to insulin, though its expression in heart muscle cells seems independent of insulin, suggesting a role as a baseline glucose transporter.
GLUT13 (HMIT) is a proton-driven myo-inositol transporter highly expressed in the brain (especially the brain stem, cerebellum, hippocampus, and hypothalamus) as well as in kidney and adipose tissue. It is primarily located intracellularly and moves to the membrane in response to depolarization or protein kinase C activation. GLUT13 is unique because it transports inositol-3-phosphate, which is critical for producing phosphatidylinositol — a key regulator of cell signaling pathways. Dysregulation of this pathway has been associated with psychiatric conditions such as bipolar disorder.
Summary Table: GLUT Classes, Tissue Distribution, and Functions
Conclusion
The vital guardians of the cellular energy supply are glucose transporters (SGLTs and GLUTs). While GLUTs let glucose to travel along its concentration gradient by facilitated diffusion, SGLTs actively transport glucose into intestine and renal cells using sodium gradients. Class I transporters (GLUT1–4) are important for insulin-regulated glucose absorption, brain energy supply, and blood glucose homeostasis. Fructose, urate, and even intracellular glucose trafficking are added to the transport capabilities by Classes II and III.
It is essential to comprehend the distribution, regulation, and function of these transporters in order to interpret diseases including diabetes, GLUT1 deficient syndrome, metabolic syndrome, and several neurological and psychiatric conditions, as well as to understand normal physiology. Nowadays, GLUT and SGLT targeting is a key component of contemporary treatment approaches, such as GLUT-targeted cancer research and SGLT2 inhibitors for diabetes.
FAQs
1. What is the difference between SGLTs and GLUTs?
SGLTs (Sodium-Glucose Linked Transporters) use sodium gradients for active glucose transport, while GLUTs transport glucose by facilitated diffusion without using energy.
2. Which GLUT is insulin dependent?
GLUT4 is the only insulin-responsive GLUT, found in muscle and adipose tissue.
3. Why does the brain mainly use GLUT3?
GLUT3 has a high affinity for glucose, ensuring constant glucose uptake even when blood glucose levels are low.
4. What is the main glucose sensor in pancreatic β-cells?
Glucokinase is the primary glucose sensor, although GLUT1/GLUT2 facilitates glucose entry into the cell.
5. Which GLUT transports fructose?
GLUT5 is the primary fructose transporter, while GLUT11 also facilitates both glucose and fructose transport.
6. How does GLUT2 regulate liver glucose metabolism?
GLUT2 enables bidirectional transport of glucose in hepatocytes, allowing glucose to enter during the fed state and exit during fasting.
7. Which GLUT is linked to uric acid transport?
GLUT9 helps transport urate and is relevant in uric acid metabolism and gout.
8. Are GLUT transporters involved in cancer?
Yes, many cancers overexpress GLUT1 to meet their high glucose demands for rapid growth.
9. What happens in GLUT1 deficiency syndrome?
There is insufficient glucose transport across the blood-brain barrier, leading to seizures, developmental delay, and neurological issues.
10. Can GLUT transporters be therapeutic targets?
Yes, targeting GLUTs (e.g., GLUT1 inhibitors) is being explored in cancer therapy, while SGLT2 inhibitors are already used in diabetes treatment.
Practice Questions
Analytical Questions
Differentiate between SGLTs and GLUTs in terms of structure, function, and energy dependence.
Explain why GLUT3 is considered the primary neuronal glucose transporter.
Discuss the significance of insulin in regulating GLUT4 translocation.
Describe how GLUT2 contributes to glucose homeostasis in the liver and pancreas.
Compare and contrast the substrate specificity of GLUT5 and GLUT11.
Critical Thinking Questions
Why might cancer cells overexpress GLUT1, and how could this be targeted therapeutically?
A patient has recurrent hypoglycemia but normal insulin secretion — how might a defect in GLUT2 contribute to this condition?
Explain why GLUT12 might be considered a "backup" glucose transporter in skeletal muscle.
How would a mutation in SGLT2 affect renal glucose handling and potentially lead to glycosuria?
Propose an experiment to measure GLUT4 translocation in muscle cells in response to insulin.
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.