Thin Layer Chromatography (TLC) of Amino Acids — Detailed Guide
Thin-layer chromatography (TLC) is a simple, yet powerful analytical technique that can be used to separate and identify amino acids present in a mixture. Because amino acids are colorless and polar, TLC requires some specific conditions — such as the use of polar solvents and special visualization reagents — to achieve good separation and clear results. This article will walk you through the principle, step-by-step procedure, explanation of each step, choice of solvent systems, and visualization techniques specifically for amino acids.
PROTOCOLS
9/18/20254 min read
Principle of TLC for Amino Acids
TLC works on the principle of differential adsorption. The plate is coated with a stationary phase (silica gel or cellulose), which is polar and can form hydrogen bonds. The mobile phase (a solvent or mixture) moves up the plate by capillary action, carrying the amino acids along.
More polar amino acids (like lysine, aspartic acid) bind more strongly to the silica layer and move more slowly.
Less polar amino acids (like phenylalanine, leucine) have weaker interactions and travel further.
This difference allows separation of amino acids into distinct spots, which can then be identified by their Rf values.
Materials Required
TLC plates (silica gel 60 or cellulose plates work well)
Sample solutions (individual amino acids or mixture, dissolved in distilled water or ethanol–water mixture)
Mobile phase solvents (e.g., butanol : acetic acid : water = 4:1:5 — a classic for amino acids)
Developing chamber (beaker + lid/watch glass)
Capillary tubes for spotting
Pencil and ruler
Ninhydrin reagent (1% in acetone or ethanol) for visualization
Hair dryer or oven (for heating plate after spraying)
Forceps, gloves, goggles
Step-by-Step Protocol
1️⃣ Preparing the Plate
Take a TLC plate and draw a baseline with a pencil about 1 cm from the bottom edge. Mark small tick marks for each sample position.
Why: Pencil is used because ink dissolves in the solvent and can interfere with the results.
2️⃣ Spotting the Samples
Use a capillary tube to apply very small, concentrated spots of each amino acid solution (or mixture) on the baseline.
Why: Concentrated, tiny spots produce sharper, more distinct bands after development. Large, diffuse spots can overlap and reduce resolution.
Allow the spots to dry completely before moving to the next step.
3️⃣ Preparing the Mobile Phase
Prepare a solvent mixture suitable for amino acids. The most commonly used is:
Butanol:AceticAcid:Water=4:1:5
Why: This solvent system is polar enough to carry amino acids up the plate but still allows them to separate based on polarity differences.
Pour the solvent into the chamber to about 0.5 cm depth. Line the chamber with filter paper wetted with the same solvent mixture to saturate the chamber. Close the chamber and allow to equilibrate for a few minutes.
Why: Chamber saturation helps produce straight, uniform solvent fronts and better resolution.
4️⃣ Developing the Plate
Place the spotted TLC plate carefully in the chamber using forceps. Ensure:
The solvent level is below the baseline (to avoid dissolving spots).
The plate stands vertically and does not touch the chamber walls.
Allow the solvent front to rise until it is ~1–2 cm from the top edge of the plate. Remove the plate and immediately mark the solvent front with a pencil.
Why: Marking quickly is important because the solvent starts to evaporate, and you need the correct distance to calculate Rf values later.
5️⃣ Drying the Plate
Let the plate air dry completely before visualization.
6️⃣ Visualizing the Spots
Since amino acids are colorless, spray the plate with ninhydrin reagent evenly. Then gently heat the plate using a hot air oven or hair dryer at ~80–100 °C for a few minutes until purple spots appear.
Why: Ninhydrin reacts with the amino group (-NH₂) of amino acids to form Ruhemann’s purple, which is easily visible.
7️⃣ Measuring and Calculating Rf Values
Measure the distance traveled by:
The solvent front (dₓ)
The center of each amino acid spot (dₛ)
Calculate Rf value using the formula:
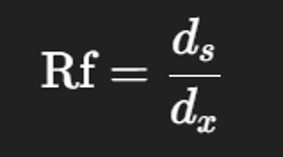
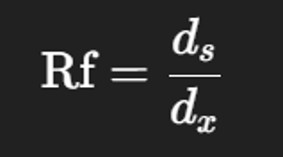
Compare the Rf values with those of known amino acids under the same conditions to identify them.
Interpretation and Expected Results
Compare the Rf values with those of known amino acids under the same conditions to identify them.
*Rf values depend on plate, solvent composition, and temperature — always run standards alongside your mixture for accurate identification.
⚠️ Troubleshooting Tips
Applications of Amino Acid TLC
Qualitative analysis: Detecting which amino acids are present in a mixture
Purity check: Ensuring only one spot appears for pure amino acid sample
Hydrolysis studies: Checking amino acid composition after protein hydrolysis
Teaching & demonstration: Simple, inexpensive way to teach chromatography principles
Frequently Asked Questions (FAQ)
1. Why is TLC useful for amino acid analysis?
TLC is a quick, inexpensive way to separate and identify amino acids. It is widely used in teaching labs to demonstrate chromatographic separation and to monitor hydrolyzed protein samples for their amino acid composition.
2. Why do we use butanol:acetic acid:water as the solvent system?
This mixture is polar enough to move amino acids but allows them to separate based on polarity. Butanol provides a slightly nonpolar phase, acetic acid helps protonate amino acids to improve migration, and water ensures solubility.
3. Can we use other solvent systems for amino acids?
Yes. Alternative solvent systems include:
Phenol:water (4:1) – good for aromatic amino acids
Ethanol:water:ammonia (70:20:10) – good for separating basic amino acids
Choice depends on the polarity of amino acids you wish to separate.
4. Why can’t we see amino acids directly on a TLC plate?
Amino acids are colorless and do not absorb visible light strongly, so they are invisible to the naked eye. This is why we use ninhydrin spray, which reacts with the amino group to give a purple color (Ruhemann’s purple).
5. What does Rf value tell us?
Rf (retention factor) is a characteristic value that helps in identification. If you run known amino acids alongside your unknown sample, you can compare Rf values and identify the components.
6. Why do we line the developing chamber with filter paper?
Lining the chamber with filter paper ensures that the air inside is saturated with solvent vapors, giving a uniform solvent front and preventing curved or “smiling” fronts.
7. Why is it important to keep spots small and concentrated?
Large or overloaded spots cause streaking, overlap with other compounds, and poor separation. Multiple tiny applications of the same sample (drying between each) work better for sharp, focused spots.
8. What if no spots are visible after spraying with ninhydrin?
The sample might be too dilute → try spotting more sample or concentrating it.
The heating might not be sufficient → warm longer (but avoid burning the plate).
Some compounds (like secondary amines or imino acids) give weak or different colors → use a stronger reagent or different visualization method.
9. How accurate is TLC for quantifying amino acids?
TLC is mainly qualitative (for identification). Quantification is possible using densitometry or digital image analysis, but techniques like HPLC are preferred for precise quantitative analysis.
10. What are common mistakes to avoid in amino acid TLC?
Dipping the baseline into solvent (spots dissolve → no result)
Not allowing chamber to equilibrate (leads to irregular front)
Using ink for baseline (dissolves and interferes)
Overheating plate after spraying (can char spots or background)
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.