The 2025 Nobel Prize in Chemistry: The “Super Sponge” Revolution in Materials Science
The 2025 Chemistry Nobel honors Susumu Kitagawa, Richard Robson, and Omar Yaghi for developing metal–organic frameworks — molecular “super sponges” that are transforming materials science, clean energy, and environmental technology.
SUSTAINABILITY & BIOENERGY
Dr. Mainak Mukhopadhyay
10/14/20254 min read
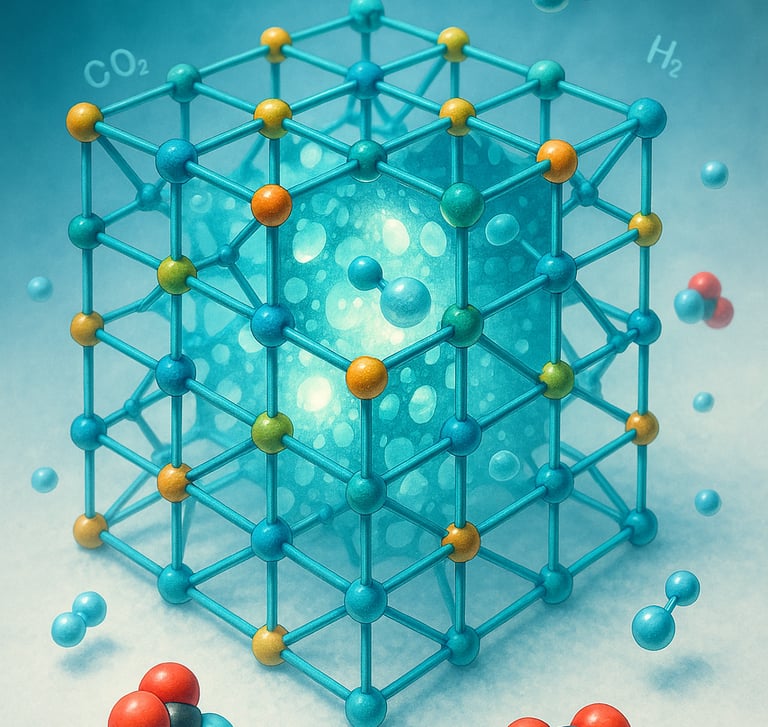
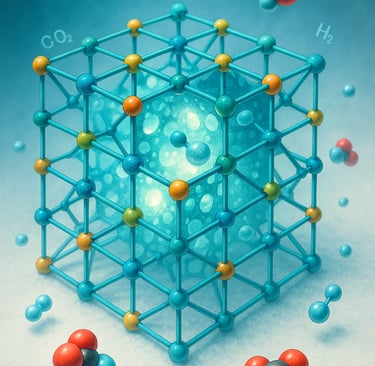
In 2025, the Nobel Prize in Chemistry was jointly awarded to Susumu Kitagawa, Richard Robson, and Omar M. Yaghi for their pioneering work on metal–organic frameworks (MOFs) — crystalline materials with immense internal surface areas and tunable porosity that behave like “super sponges” at the molecular scale [1].
This landmark recognition celebrates not just structural ingenuity but a profound shift in chemical design thinking — from observing natural structures to building materials atom by atom with predetermined architecture and function.
From Coordination Chemistry to Framework Design
The origins of MOFs can be traced to the evolving field of coordination chemistry in the late twentieth century. During the 1980s, Richard Robson (University of Melbourne) and colleagues proposed that coordination compounds could extend beyond discrete complexes to form infinite network structures through metal–ligand bonding [2]. This idea — that metal ions could serve as “nodes” connected by organic “linkers” — laid the conceptual groundwork for later framework materials.
Susumu Kitagawa (Kyoto University) advanced this concept by exploring porous coordination polymers and their dynamic behaviors. His studies revealed that such frameworks could “breathe,” expanding and contracting in response to external stimuli like gas pressure or temperature [3]. Kitagawa’s insights showed that porosity was not a static trait but a reversible, controllable phenomenon, paving the way for adaptive and responsive materials.
Meanwhile, Omar Yaghi (University of California, Berkeley) introduced the term metal–organic frameworks in the mid-1990s and developed a systematic approach he called reticular chemistry [4]. By combining carefully chosen metal clusters with rigid organic linkers, Yaghi’s team built crystalline frameworks with record-breaking porosity and stability. His work established MOFs as a distinct and versatile class of materials, inspiring laboratories worldwide to explore their potential.
What Makes MOFs Extraordinary
Metal–organic frameworks are solids that paradoxically contain vast internal voids. Within a single gram of some MOFs, the total surface area can exceed 6,000 m² — roughly the size of a football field [5]. This extreme porosity allows them to capture, store, and release gas molecules such as carbon dioxide, hydrogen, or methane with remarkable efficiency.
What makes MOFs truly unique is their tunability. Chemists can design frameworks to selectively interact with specific molecules by altering metal centers or organic linkers. As a result, MOFs serve as customizable platforms for gas storage, separations, catalysis, sensing, and even drug delivery [6].
Yaghi’s group, for instance, demonstrated that certain MOFs can adsorb and release hydrogen at moderate pressures, offering a potential route to safe, efficient fuel storage [7]. Others have developed CO₂-selective frameworks that could play key roles in carbon capture and sequestration technologies [8].
Challenges on the Road to Application
Despite their promise, MOFs face challenges that remain the focus of active research. Many early frameworks were unstable in humid or acidic environments, limiting practical deployment [9]. Researchers are now developing new generations of MOFs with enhanced robustness, exploring green synthesis routes, and employing computational screening to predict which structures will combine stability with high performance.
Another obstacle is scalability. Synthesizing MOFs at the industrial scale requires precise control over crystal growth and purity while keeping costs manageable. Integration into usable forms — powders, membranes, coatings — is a nontrivial task that demands collaboration between chemists, engineers, and materials scientists.
Yet, these challenges are also opportunities. The field’s rapid progress suggests that MOFs are transitioning from laboratory curiosities to practical materials capable of addressing global issues such as climate change, clean energy, and sustainable manufacturing.
Why This Nobel Matters for Students and Researchers
For today’s students and early-career scientists, the Nobel recognition of MOFs offers several important lessons.
First, it exemplifies the power of interdisciplinary thinking. The success of MOF research lies at the intersection of inorganic chemistry, organic synthesis, materials science, and physics. Understanding and manipulating matter at this interface have led to properties that no single discipline could achieve alone.
Second, the MOF story shows how creativity and design can drive discovery. Chemistry is often seen as an experimental science, but in MOF design, it becomes an architectural one — where structure and function are planned with mathematical precision.
Finally, it reminds us that fundamental science can yield transformative applications. The frameworks that began as academic explorations in crystal engineering are now being integrated into prototypes for gas storage tanks, water harvesters, and catalytic reactors.
For undergraduate students, MOFs offer an accessible entry point into modern materials chemistry — bridging molecular bonding with macroscopic function. For postgraduate researchers, they represent fertile ground for innovation in sustainability and nanotechnology. And for the scientific community at large, this Nobel highlights chemistry’s capacity to solve pressing environmental challenges through elegant molecular design.
Looking Toward the Future
The next chapter of MOF research may involve bio-MOFs, combining organic frameworks with biomolecules for medical and catalytic applications, or conductive MOFs, enabling electronic and quantum materials. Researchers are also exploring hybrid composites that blend MOFs with polymers or graphene for improved strength and function.
As machine learning and computational chemistry mature, scientists can now screen millions of hypothetical frameworks in silico, predicting those with optimal pore sizes or chemical affinities long before laboratory synthesis. This synergy between computation and experiment is accelerating discovery at unprecedented rates [10].
Conclusion
The 2025 Nobel Prize in Chemistry celebrates more than three scientists — it honors a collective shift in how chemists think about matter, design, and possibility. By showing that solids can be engineered as molecular sponges, Kitagawa, Robson, and Yaghi opened a path toward sustainable technologies that may define twenty-first-century chemistry.
Their legacy will inspire students and researchers alike to continue asking bold questions: What else can we build at the atomic scale? and How might it change the world around us?
References
Nature News. Chemistry Nobel for scientists who developed massively porous “super sponge” materials. Nature, 2025. https://www.nature.com/articles/d41586-025-03195-1
Hoskins, B. F., Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. J. Am. Chem. Soc. (1990).
Kitagawa, S., Kitaura, R., Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. (2004).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature (2003).
Furukawa, H., Cordova, K. E., O’Keeffe, M., Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science (2013).
Li, J.-R., Kuppler, R. J., Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. (2009).
Rosi, N. L. et al. Hydrogen storage in microporous metal–organic frameworks. Science (2003).
Sumida, K. et al. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. (2012).
Burtch, N. C., Jasuja, H., Walton, K. S. Water stability and adsorption in metal–organic frameworks. Chem. Rev. (2014).
Moghadam, P. Z. et al. Computer-aided discovery of MOFs for gas storage. Nat. Commun. (2018).
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.