Pyruvate Dehydrogenase Complex (PDC): Step-by-Step Mechanism, Enzymes, Cofactors, and Regulation Explained
Discover the detailed reaction mechanism of the Pyruvate Dehydrogenase Complex (PDC). Learn how E1, E2, and E3 enzymes convert pyruvate into acetyl-CoA through TPP, lipoic acid, FAD, NAD⁺, and CoA cofactors, with full explanations, regulation insights, and clinical relevance for postgraduate biochemistry students.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
10/12/20259 min read
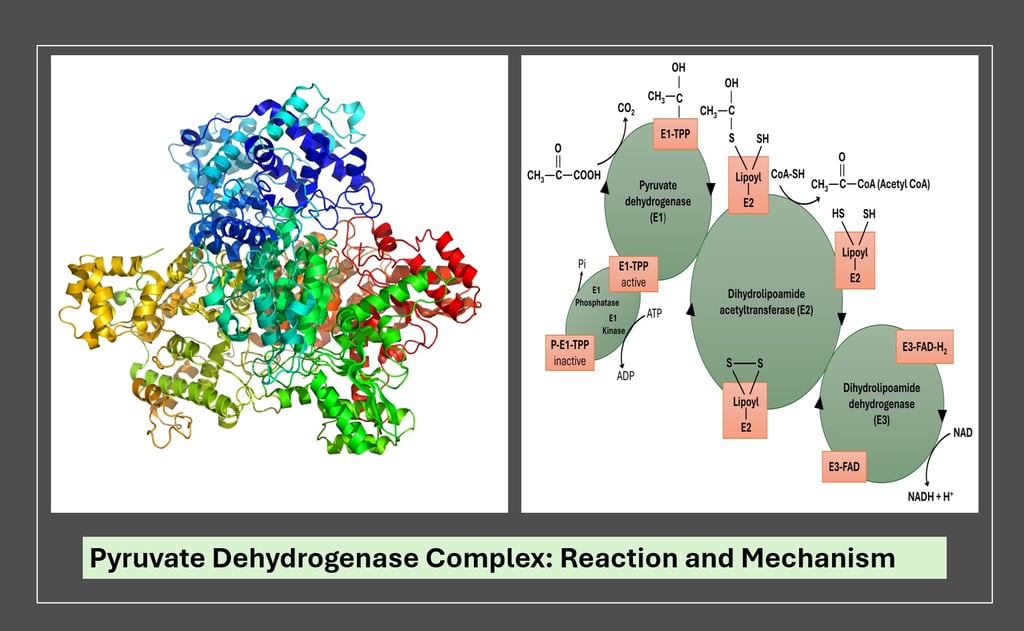
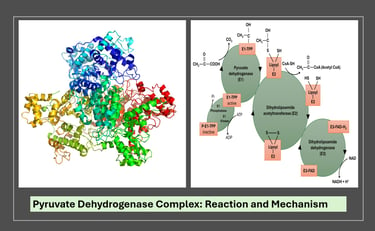
The Pyruvate Dehydrogenase Complex (PDC) is a multienzyme complex that catalyzes the oxidative decarboxylation of pyruvate to form acetyl-CoA, which then enters the citric acid (TCA) cycle. This reaction acts as a crucial metabolic link between glycolysis and the TCA cycle, thereby regulating the flow of carbon from carbohydrates into aerobic energy metabolism.
Overall Reaction:
This reaction is irreversible under physiological conditions and represents a key commitment step to aerobic metabolism.
Structural Organization
The PDC is a large multienzyme complex (~9.5 MDa in mammals), consisting of multiple copies of three core enzymes:
Stoichiometry: In mammalian mitochondria, the PDC core consists of 60 E2 subunits forming a cubic or dodecahedral scaffold surrounded by multiple copies of E1 and E3.
Cofactors and Their Roles
Step-by-Step Reaction Mechanism
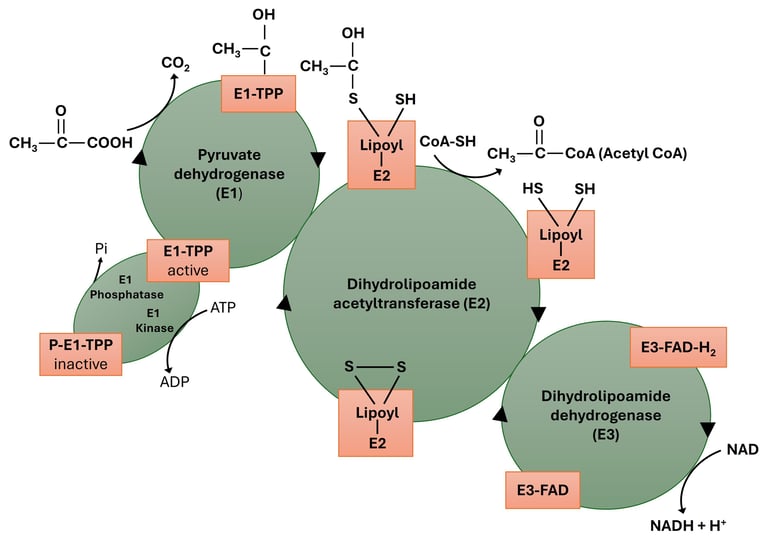
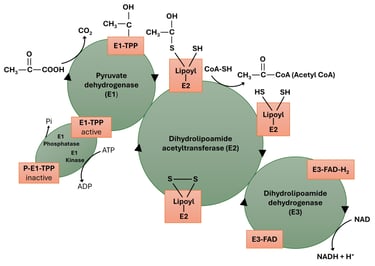
Step 1 — Decarboxylation of pyruvate by E1 (pyruvate dehydrogenase; TPP-dependent)
What happens (big picture)
E1 removes CO₂ from pyruvate and forms a hydroxyethyl-TPP adduct. This is a TPP-catalyzed decarboxylation: TPP’s thiazolium ring stabilizes the carbanion/enamine-like intermediate (acts as an “electron sink”).
Detailed chemical events
Activation of TPP: In the E1 active site, the thiazolium C2 of TPP is deprotonated (or exists as a stabilized ylide/carbanion) by an active-site base (often a glutamate or histidine residue nearby). This produces the nucleophilic C2 carbanion on the thiazolium ring.
Nucleophilic attack on pyruvate: The TPP carbanion attacks the carbonyl carbon of pyruvate to form a covalent addition intermediate (a pre-decarboxylation adduct).
Decarboxylation: The tetrahedral-like intermediate collapses and releases CO₂, leaving a resonance-stabilized hydroxyethyl-TPP (an enamine-type species). The positive charge of the thiazolium stabilizes the negative charge generated in the process.
Resonance stabilization & geometry: The hydroxyethyl fragment is positioned on the thiazolium ring in such a way that electron density is delocalized across the thiazolium — this stabilization is crucial; without TPP, decarboxylation would be energetically unfavorable.
Stereochemistry: The newly formed hydroxyethyl moiety at the TPP center is oriented so that it can be delivered to the lipoamide arm of E2 in a defined stereochemical manner (E1 controls approach geometry).
Active-site/structural notes
TPP binding pocket uses conserved residues to orient both TPP and pyruvate.
E1 is typically a heterotetramer (α₂β₂) in eukaryotes; the active site is at α–β interfaces.
The distance between the hydroxyethyl-TPP and the lipoamide arm is minimized by the architecture of the E1–E2 interface so that transfer to lipoamide is efficient.
Step 2 — Oxidation of the hydroxyethyl group and formation of acetyllipoamide (transfer to E2 lipoamide)
What happens (big picture)
The hydroxyethyl group on TPP is oxidized to an acetyl while electrons reduce the disulfide of the lipoamide (—S—S— → —SH HS—). The acetyl group becomes covalently attached to one sulfur of the lipoamide (forming acetyllipoamide, i.e., S-acetyl-dihydrolipoamide?—terminology: acetyllipoamide usually means the oxidized lipoamide bearing an acetyl group on one sulfur).
Detailed chemical events
Nucleophilic attack by lipoamide: The oxidized lipoamide disulfide (—S—S—) attached to the long flexible E2 lipoamide arm approaches the hydroxyethyl-TPP. One of the lipoamide sulfurs performs a nucleophilic attack on the hydroxyethyl carbon attached to TPP (or an activated form thereof).
Transfer and oxidation: During transfer, the hydroxyethyl group is oxidized (two electrons removed) as it forms a thioester (S–C=O) on lipoamide — generating S-acetyl-lipoamide (acetyllipoamide) and simultaneously reducing the disulfide bond of lipoamide to the dithiol (dihydrolipoamide) (—SH HS—). Mechanistically this is a disulfide exchange/thiol attack plus hydride/electron transfer from the hydroxyethyl moiety to the disulfide, coupled to release of TPP back to its resting form.
Regeneration of free TPP: The electron redistribution returns TPP to its catalytically active form (carbanion stabilized by thiazolium).
Key mechanistic ideas
The transfer is essentially a two-electron oxidation of the hydroxyethyl group to an acetyl group, with the electrons accepted by the lipoamide disulfide.
The lipoamide arm acts as both substrate acceptor and mobile carrier — the “swinging arm” physically brings the acyl moiety to the E2 catalytic site and then to E3.
Structural notes
Lipoamide is covalently attached to a lysine of E2 via an amide linkage; the arm length (~16 Å or more) is tuned to reach E1, E2 active-center positions, and E3.
The E1–E2 interface aligns reactive groups to minimize solvent exposure and to prevent uncoupled oxidation events.
Step 3 — Transfer of acetyl group from acetyllipoamide to CoA (E2 — dihydrolipoyl transacetylase)
What happens (big picture)
E2 catalyzes nucleophilic attack by CoA on the thioester linkage (acetyllipoamide), transferring the acetyl group onto CoA to form acetyl-CoA and leaving lipoamide in the reduced dithiol (dihydrolipoamide) form.
Detailed chemical events
Orienting the substrates: The E2 active site binds CoA and positions its thiol (CoA–SH) close to the acetyllipoamide thioester. Key residues in E2 help deprotonate CoA-SH slightly or stabilize the thiolate transition state (a conserved histidine/lysine may act to lower CoA thiol pKa locally).
Nucleophilic attack: The CoA thiol attacks the carbonyl carbon of the acetyllipoamide thioester in a standard thiol-thioester exchange mechanism. This forms a tetrahedral intermediate at the carbonyl carbon.
Collapse to product: The tetrahedral intermediate collapses, releasing dihydrolipoamide (—SH HS—) and forming acetyl–CoA (thioester linkage on CoA). No net redox occurs in this step — the acetyl group is simply transferred.
Product release: Acetyl–CoA diffuses away (or is channeled) to the TCA cycle; dihydrolipoamide remains attached to E2 awaiting reoxidation.
Catalytic notes
E2’s active site environment stabilizes the transition state and provides proximity; the energetic favorability of acetyl–CoA formation depends on local concentrations and on subsequent consumption of acetyl–CoA in TCA, shifting reaction forward.
Transfer is essentially reversible chemically but in the cellular context acetyl–CoA is swiftly used → pushes forward.
Structural/functional roles of E2
E2 forms the structural core (oligomeric scaffold) that organizes many E1 and E3 copies.
The mobility of the lipoamide arm allows one lipoamide to visit E1, E2 active sites repeatedly.
Step 4 — Reoxidation of dihydrolipoamide by E3 (dihydrolipoyl dehydrogenase; FAD-dependent)
What happens (big picture)
E3 catalyzes the oxidation of the reduced dithiol form of lipoamide back to the oxidized disulfide, using FAD as the immediate electron acceptor (forming FADH₂). This regenerates the lipoamide swinging arm so it can accept a new acyl group.
Detailed chemical events
Dithiol binds E3: The reduced dihydrolipoamide (—SH HS—) approaches the active-site disulfide of E3 (often a Cys–Gly–Pro–Cys motif). The active-site disulfide of E3 is the immediate acceptor for electrons.
Thiolate attack/disulfide exchange: One of the thiolates from lipoamide attacks the disulfide in E3, forming a mixed disulfide and releasing one cysteine thiolate on E3. Then a second attack resolves the mixed disulfide to fully reoxidize lipoamide to the internal disulfide (—S—S—) and reduce the E3 active-site disulfide to dithiol (—SH HS—) — net result: electrons transferred to the E3 active-site cysteines.
Electron transfer to FAD: The reduced E3 active site passes two electrons to FAD, reducing it to FADH₂; this occurs via hydride-like electron transfers within the enzyme. Mechanistically, the two electrons move from the cysteine thiols to the flavin isoalloxazine ring.
Consequence: The lipoamide is now reoxidized and can re-enter step 2 for another catalytic cycle. FADH₂ remains bound to E3 until step 5.
Active-site chemistry notes
The E3 active site contains a redox-active disulfide (Cys–X–X–Cys) and residues that stabilize transition states.
The mechanism proceeds via disulfide exchange rather than direct hydride transfer from lipoamide to FAD; E3’s disulfide acts as an electron shuttle between lipoamide and FAD.
Step 5 — Reoxidation of FADH₂ by NAD⁺ (E3: FAD acts as mediator to NAD⁺)
What happens (big picture)
Electrons from FADH₂ are transferred to NAD⁺, forming NADH + H⁺. This completes the electron-relay chain and regenerates oxidized FAD ready for another cycle.
Detailed chemical events
Hydride transfer: FADH₂ donates a hydride equivalent (H⁻) to NAD⁺ (specifically to the nicotinamide ring carbon), resulting in NADH and restoring FAD. This is a classical flavin-to-NAD⁺ hydride transfer step.
Release: NADH diffuses away to the mitochondrial matrix where it donates its electrons to Complex I of the electron transport chain (or participates in other redox reactions).
Catalytic turnover: With FAD reoxidized, E3 is ready for the next cycle of lipoamide reoxidation.
Kinetic/regulatory notes
The binding affinities and relative concentrations of NAD⁺/NADH influence the redox balance. High NADH/NAD⁺ ratio inhibits reoxidation and therefore blocks PDC turnover (feedback inhibition).
The E3-catalyzed FAD ↔ NAD⁺ step is often fast and efficient; however, in vivo NADH levels strongly influence net flux through the PDC.
Active-site residues & catalysis — enzyme-by-enzyme highlights
E1 (pyruvate dehydrogenase; TPP-dependence)
Key catalytic roles: provide TPP, orient pyruvate, stabilize leaving group (CO₂), and position hydroxyethyl-TPP for transfer.
Important residues: conserved residues that stabilize TPP (e.g., histidine/arginine to bind pyrophosphate), acidic residues to abstract proton and orient substrate.
E2 (dihydrolipoyl transacetylase)
Key catalytic residues: residues in E2 coordinate CoA and stabilize transition states; may include histidine/serine to orient CoA thiol.
Structural role: E2 core oligomerization provides scaffold; number of E2 subunits determines geometry and spacing for E1/E3 assembly.
E3 (dihydrolipoyl dehydrogenase; FAD-dependent)
Key motifs: conserved Cys–Gly–Pro–Cys (or similar) active-site disulfide motif; FAD-binding domain with conserved glycine-rich motif for FAD binding.
Catalytic residues: residues that position NAD⁺ for hydride acceptance.
Concise summary of each step (one-line each)
E1 (TPP) — decarboxylates pyruvate, forms hydroxyethyl-TPP (stabilized carbanion).
E1→E2 — hydroxyethyl is oxidized and transferred to lipoamide, forming acetyllipoamide and reducing the lipoamide disulfide.
E2 — CoA thiol attacks acetyllipoamide to yield acetyl–CoA and reduced dihydrolipoamide.
E3 — reduced lipoamide is reoxidized via a disulfide exchange to E3 active-site disulfide, reducing FAD to FADH₂.
E3 (FAD→NAD⁺) — FADH₂ transfers electrons to NAD⁺ producing NADH; FAD is regenerated.
Regulation of the Pyruvate Dehydrogenase Comple
The PDC is tightly regulated through allosteric control and covalent modification, ensuring metabolic balance.
A. Allosteric Regulation
High energy charge (ATP, NADH, acetyl-CoA) → Inhibition
Low energy charge (ADP, NAD⁺, pyruvate) → Activation
B. Covalent Regulation
The E1 subunit is inactivated by phosphorylation and activated by dephosphorylation
Frequently Asked Questions (FAQ)
1. What is the function of the Pyruvate Dehydrogenase Complex (PDC)?
The PDC catalyzes the oxidative decarboxylation of pyruvate to form acetyl-CoA, linking glycolysis to the citric acid (TCA) cycle. This reaction commits glucose-derived carbon to aerobic energy metabolism.
2. Why is the PDC reaction irreversible?
The reaction releases CO₂, which diffuses out of the mitochondria, making the decarboxylation step thermodynamically irreversible under physiological conditions. Hence, acetyl-CoA cannot be converted back to pyruvate in animals.
3. Where is the PDC located in eukaryotic cells?
It is found in the mitochondrial matrix, allowing immediate transfer of acetyl-CoA into the TCA cycle for energy production.
4. Which vitamins are required for PDC activity?
The complex requires five cofactors derived from vitamins:
TPP — from Vitamin B₁ (Thiamine)
FAD — from Vitamin B₂ (Riboflavin)
NAD⁺ — from Vitamin B₃ (Niacin)
CoA — from Vitamin B₅ (Pantothenic acid)
Lipoic acid — synthesized within the body
5. How is the PDC regulated?
It is regulated by:
Allosteric effectors: Inhibited by ATP, NADH, Acetyl-CoA; activated by ADP, CoA-SH, pyruvate.
Covalent modification: E1 is inactivated by phosphorylation (via PDK) and reactivated by dephosphorylation (via PDP).
6. What happens if PDC is deficient?
PDC deficiency or cofactor deficiency (e.g., thiamine deficiency) leads to pyruvate accumulation and lactic acidosis, causing neurological symptoms due to impaired ATP generation in brain tissues.
7. What role does calcium play in PDC regulation?
During muscle contraction, Ca²⁺ activates pyruvate dehydrogenase phosphatase (PDP), which removes inhibitory phosphate from E1, increasing PDC activity to meet energy demands.
8. How is PDC linked to the TCA cycle and Electron Transport Chain?
The acetyl-CoA generated enters the TCA cycle, producing NADH and FADH₂ that deliver electrons to the electron transport chain, ultimately driving ATP synthesis.
9. What is the clinical relevance of arsenite poisoning in PDC?
Arsenite binds to lipoamide’s thiol groups in E2, inhibiting acetyl group transfer and leading to metabolic blockade—mimicking thiamine deficiency symptoms.
10. Is the Pyruvate Dehydrogenase Complex similar in bacteria and humans?
Yes, but the organization differs: bacteria often have smaller PDCs with similar enzyme functions, localized in the cytosol instead of mitochondria.
Practice Questions for Different Levels
🔹 Level 1 — Basic Recall Questions
Write the overall reaction catalyzed by the pyruvate dehydrogenase complex.
Name the three enzymes of the PDC and their respective cofactors.
Which cofactor is derived from vitamin B₁?
In which cellular compartment is the PDC located in eukaryotes?
Name the products formed in the PDC reaction.
What type of reaction does PDC perform: oxidation, reduction, or decarboxylation?
Which step of the PDC reaction releases CO₂?
Why is lipoic acid called a “swinging arm”?
🔹 Level 2 — Conceptual / Intermediate Questions
Explain how the hydroxyethyl group is transferred from TPP to lipoamide.
Describe how FAD and NAD⁺ participate in the regeneration of oxidized lipoamide.
Discuss the importance of substrate channeling in the PDC.
How does thiamine deficiency affect pyruvate and lactate levels in blood?
Compare the effects of high NADH/NAD⁺ ratio on glycolysis and PDC activity.
Why is PDC considered the “gatekeeper” between anaerobic and aerobic metabolism?
Explain the difference between E1 phosphorylation and allosteric inhibition by NADH.
🔹 Level 3 — Analytical / Application Questions
Trace the path of electrons from pyruvate to NADH within the PDC.
Predict what happens to PDC activity when muscle cells switch from resting to active contraction.
Explain how arsenite poisoning inhibits PDC using its mechanistic step.
How does PDC integrate with fatty acid synthesis in the fed state?
Outline how the PDC reaction links carbohydrate metabolism to lipid metabolism.
Discuss how the accumulation of acetyl-CoA and NADH affects both PDC and TCA cycle activity.
Compare the structural organization of PDC with α-ketoglutarate dehydrogenase complex.
🔹 Level 4 — Advanced / Critical Thinking Questions
The PDC reaction is often described as “oxidative decarboxylation.” Explain each term mechanistically and discuss the thermodynamic basis for irreversibility.
Design an experiment to measure PDC activity in isolated mitochondria. Include substrate, cofactor, and detection method.
How would a mutation in the E3 subunit affect redox balance in mitochondria?
Propose how phosphorylation/dephosphorylation cycles of PDC coordinate with insulin and glucagon signaling in liver cells.
Consider a patient with lactic acidosis and normal lactate dehydrogenase levels—how would you biochemically distinguish a PDC defect?
Discuss the evolutionary advantage of organizing the three enzymes into a multienzyme complex rather than separate proteins.
Suppose a novel cofactor analog is designed to inhibit TPP binding competitively — predict the effect on energy metabolism in neuronal cells.
🧩 Optional Extension: Short-Answer / Exam Type Questions
Write short notes on:
a. Role of thiamine pyrophosphate in oxidative decarboxylation
b. Substrate channeling in multienzyme complexes
c. Regulation of PDC by phosphorylation
d. Clinical effects of arsenite on lipoamide functionDifferentiate between:
E1, E2, and E3 in terms of function and cofactors
PDC and α-Ketoglutarate dehydrogenase complex
Allosteric regulation and covalent modification of PDC
Numerical/Conceptual type:
If one molecule of glucose produces 2 pyruvate molecules, how many NADH are formed by PDC per glucose oxidized?
→ Answer: 2 NADH per glucose (one per pyruvate).
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.