Mirror-Image Molecular Biology: Science, Promise, and Responsibility
Explore mirror-image molecular biology — the science of flipping life’s molecules into their mirror forms. Learn about chirality, stereoisomerism, potential applications in medicine, sustainability, and the ethical risks of creating mirror organisms.
MICROBIOLOGY & MOLECULAR BIOLOGY
Dr. Mainak Mukhopadhyay
9/18/20255 min read
Life, as we know it, is not only chemical but also profoundly asymmetric. Every living organism on Earth uses molecules with a consistent “handedness” — a phenomenon known as homochirality. But what if we could build life’s molecules as perfect mirror images? Could we create a parallel biological world with flipped DNA, proteins, and enzymes — and if so, should we?
This is the frontier of mirror-image molecular biology, a field that promises new medicines, materials, and insights into life’s origins — yet also raises profound biosafety and ethical questions.
Chirality and Stereoisomerism: Life’s Hidden Handedness
To understand the idea of “mirror life,” it’s crucial to first grasp chirality. Chirality describes objects or molecules that cannot be superimposed on their mirror images — much like our left and right hands.
Molecules that are mirror images are called enantiomers, a type of stereoisomer.
Life on Earth almost exclusively uses L-amino acids (left-handed) to build proteins and D-sugars (right-handed) in DNA and RNA.
This uniform selection is called homochirality, and its origin is still a major scientific mystery (Blackmond, 2022).
Mirror-image molecular biology explores the opposite set of molecules — right-handed amino acids and left-handed nucleic acids — to build biological systems that are structurally inverted compared to natural life (Young et al., 2019).
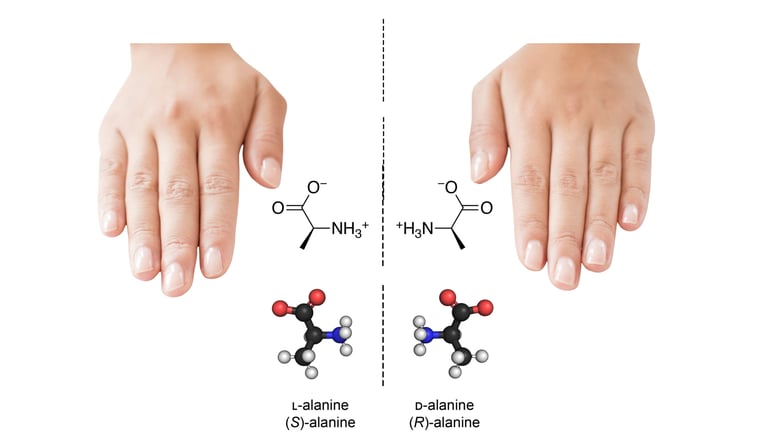
Figure 1: Chirality explained — left- and right-handed amino acids as mirror images, just like our hands.
Flipping Life’s Chemistry
Creating mirror-image life is an ambitious attempt to rebuild the central dogma of biology in reverse. Life’s information flow — DNA → RNA → protein — depends on molecules with precise 3D shapes that recognize one another like pieces of a puzzle. Reconstructing this flow using mirror molecules means flipping the entire biochemical puzzle.
Researchers have made impressive progress:
Mirror Nucleic Acids: Chemists have synthesized left-handed DNA and RNA strands, known as L-oligonucleotides or spiegelmers, which store genetic information but resist degradation by natural enzymes — a property that makes them promising for therapeutic use (Oberthür et al., 2015; Young et al., 2019).
Mirror Enzymes: Mirror versions of key enzymes, including DNA ligases and polymerases, have been synthesized chemically and shown to function, enabling mirror-PCR and mirror DNA manipulation (Weidmann et al., 2019; Pech et al., 2017).
Mirror Ribosomes: Recent breakthroughs have produced mirror fragments of ribosomal RNA and partial mirror ribosome components, hinting at the eventual possibility of mirror translation systems (Xu et al., 2022).
Yet, building a fully functional mirror organism is far from reality. Scientists must still replicate all cellular components — proteins, membranes, cofactors, metabolites — and ensure they assemble into a coherent, self-sustaining system (Zhao et al., 2014).
This research is more than just synthetic chemistry — it is a deep test of whether life could be rebuilt from first principles. It raises profound questions:
Could life have evolved with opposite chirality?
Would mirror life interact with natural life at all — or remain entirely separate?
And should we deliberately create such a parallel biology?
Potential Benefits of Mirror Life
Even without full mirror organisms, partial systems hold promise for:
Medicine & Therapeutics: Mirror peptides and nucleic acids resist enzymatic breakdown, making them stable drug candidates with prolonged effects (Liu et al., 2023).
Diagnostics: L-nucleic acids are ideal for biosensors, as they are not degraded by nucleases and avoid background interference.
Sustainability: Mirror enzymes could break down persistent pollutants or plastics, remaining active longer in harsh environments.
Nutrition: Mirror sugars could provide non-caloric sweetness, as natural metabolic enzymes cannot process them.
Astrobiology: Studying mirror systems sheds light on why life on Earth “chose” one handedness and guides our search for alien biochemistry (Blackmond, 2022).
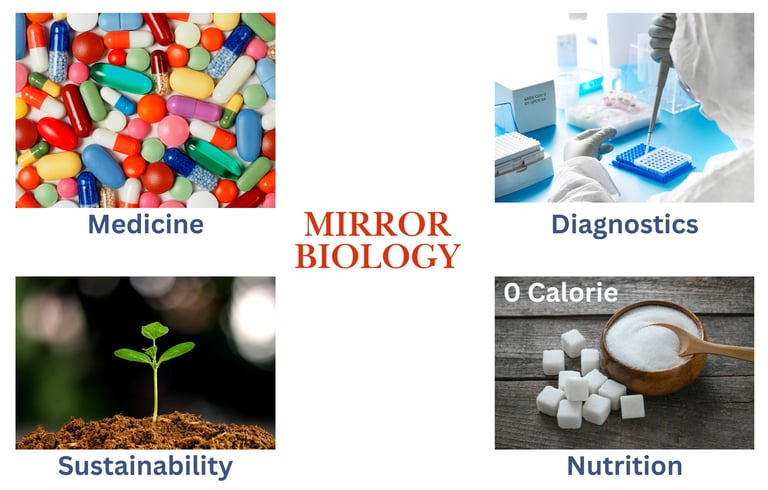
Figure 2: Applications of Mirror Biology — Four key opportunities: medicine, diagnostics, sustainability, and nutrition.
Risks and Ethical Concerns
The same features that make mirror life attractive also make it potentially hazardous:
Immune Evasion: Mirror pathogens might evade immune detection entirely.
Ecological Disruption: If mirror organisms were released, they might not be susceptible to natural predators or degradation pathways.
Dual-Use Risks: Tools developed for beneficial applications could be misused.
Some experts have called for a temporary moratorium on research aimed at self-replicating mirror organisms until governance frameworks and safety measures are in place (Kuiken & Lentzos, 2024).
Global Scientific Debate
Mirror-image molecular biology is no longer a niche curiosity — it is becoming a field of global significance, prompting scientists, ethicists, and policymakers to take notice. The central debate is not just scientific (“Can we do it?”) but also philosophical and regulatory (“Should we do it?”).
Over the past few years, several international workshops have brought together experts in synthetic biology, bioethics, and security studies to discuss the implications of creating mirror organisms. These discussions focus on:
Biosafety Standards: Current containment practices were designed for conventional pathogens. Would mirror organisms, which may resist natural degradation, require stricter containment and disposal measures?
Biosecurity and Dual-Use Risks: Could mirror molecular tools be exploited to create immune-evasive biothreats? Policymakers are considering frameworks similar to those developed for gain-of-function research and gene drive technologies (Kuiken & Lentzos, 2024).
Regulatory Gaps: Most national biosafety guidelines (e.g., NIH Guidelines, EU Directive 2009/41/EC) do not yet explicitly address synthetic life with opposite chirality, leaving oversight ambiguous.
Public Engagement: Like CRISPR, this technology touches on deep societal questions — about what it means to create life, about consent, and about ecological stewardship. Public dialogue is considered crucial to avoid a purely technocratic decision-making process.
Some experts have even suggested a temporary moratorium on research aimed at producing self-replicating mirror organisms until a global consensus is reached on risk mitigation strategies (Zhu, 2025). Others argue that controlled progress is essential — halting research could slow down the development of beneficial applications, such as stable mirror-based drugs or pollution-degrading enzymes.
What is emerging is a vision for responsible innovation: a roadmap that allows exploration of mirror biology’s potential while maintaining rigorous oversight, open data sharing, and coordinated governance between nations. Just as the Asilomar Conference on Recombinant DNA (1975) shaped modern biosafety, many researchers hope today’s conversations will lay the foundation for a global framework guiding this new and powerful technology.
Conclusion: Reflecting on Life’s Mirror
Mirror-image molecular biology challenges our understanding of chemistry, evolution, and life itself. It has the potential to transform medicine, environmental science, and synthetic biology — but it also forces us to think about the kind of future we want to build.
As this field advances, careful oversight, open public dialogue, and international collaboration will be crucial. The reflection we create must be one that illuminates new scientific possibilities — not one that casts an irreversible shadow.
References
Blackmond, D. G. (2022). The origin of biological homochirality. Cold Spring Harbor Perspectives in Biology, 14(6), a040949.
Kuiken, T., & Lentzos, F. (2024). Governance of synthetic biology and dual-use research. Science, 386(6651), 121–124.
Liu, H., et al. (2023). Mirror-image proteins for drug design: stability and therapeutic potential. Nature Reviews Drug Discovery, 22, 801–819.
Oberthür, D., et al. (2015). Crystal structure of a mirror-image L-RNA aptamer in complex with a protein. Nature Communications, 6, 6923.
Pech, A., et al. (2017). A thermostable D-polymerase for mirror-image PCR. Nature Chemistry, 9, 771–778.
Weidmann, J., et al. (2019). Synthesis of an enzymatically active mirror DNA ligase. Cell Chemical Biology, 26(5), 645–651.
Xu, Y., et al. (2022). Mirror-image T7 transcription of chirally inverted ribosomal components. Science, 378, 1225–1232.
Young, B. E., et al. (2019). Mirror-image oligonucleotides: history and emerging applications. Molecular Therapy - Nucleic Acids, 17, 486–500.
Zhao, L., et al. (2014). Mirror-image proteins: synthesis and applications. Chemical Society Reviews, 43, 6597–6620.
Zhu, T. (2025). Mirror of the unknown: should research on mirror-image molecular biology be stopped? Nature. https://doi.org/10.1038/d41586-025-02912-0
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en