Healing Bones, Muscles, and Joints Through Nanobiology: The Promise of Exosome-Based Regeneration
Discover how engineered exosomes are revolutionizing regenerative medicine by healing bones, muscles, and cartilage through nanobiology and biomaterial science
NANOTECHNOLOGY
Dr. Mainak Mukhopadhyay
10/10/20254 min read
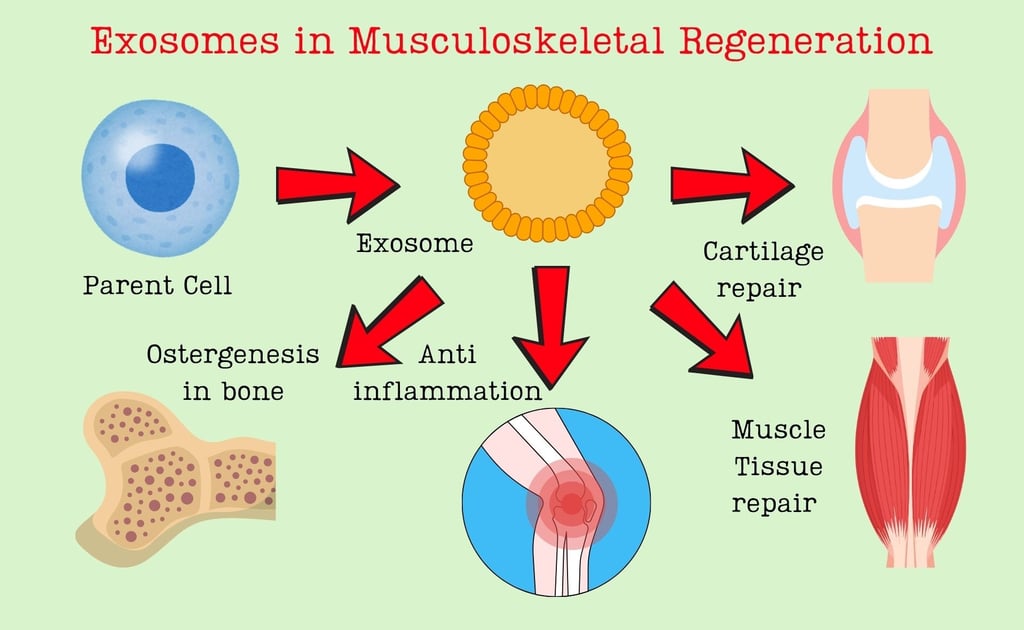
Introduction: Rethinking Regeneration in Musculoskeletal Disorders
The musculoskeletal system forms the framework of our body—bones give structure, muscles provide movement, tendons and ligaments ensure coordination, and cartilage cushions joints. When these tissues are damaged due to trauma, aging,2 or diseases such as osteoarthritis and osteoporosis, the healing process is often slow or incomplete. Traditional treatments like metal implants, bone grafts, and anti-inflammatory drugs help manage symptoms but rarely achieve true biological regeneration.
In recent years, scientists have begun exploring the body’s own communication systems for repair. One of the most promising discoveries is the therapeutic use of exosomes—tiny extracellular vesicles that cells naturally release to communicate with one another. The landmark review by Zehui Lv and colleagues, published in ACS Nano (2025), highlights how exosomes can be engineered and combined with biomaterials to promote musculoskeletal regeneration. Let’s dive deeper into what makes these nano-sized particles so revolutionary.
What Are Exosomes? Nature’s Tiny Messengers
Exosomes are nanometer-scale vesicles, typically 30–150 nm in diameter, enclosed by a lipid bilayer membrane. They originate within specialized compartments of cells known as multivesicular bodies (MVBs). When MVBs fuse with the plasma membrane, exosomes are released into the extracellular space, carrying a rich cargo of proteins, lipids, messenger RNAs (mRNAs), and microRNAs (miRNAs).
These vesicles act as biological couriers, transferring genetic and biochemical information from one cell to another. Through this exchange, exosomes can influence cell behavior—stimulating growth, suppressing inflammation, or initiating repair mechanisms.
Why Are Exosomes Attractive for Therapeutic Use?
Compared to stem cell or whole-cell therapies, exosomes offer several key advantages:
Safety: They cannot divide or mutate, minimizing the risk of tumor formation.
Stability: Exosomes are more resistant to environmental stress and easier to store long-term.
Biocompatibility: Being naturally secreted, they are well tolerated by the immune system.
Intrinsic targeting: Exosomes carry surface markers that guide them to specific tissues, such as bone or cartilage.
In simple terms, exosomes act like “biological nanodrugs” that deliver precise molecular messages to trigger healing, without the risks associated with live cell therapy.
Engineering Exosomes: Enhancing Their Therapeutic Potential
Naturally produced exosomes already have regenerative abilities, but researchers are improving them through bioengineering and bioprocess optimization. Lv et al. describe four main strategies:
Preconditioning Parent Cells
The conditions under which donor cells are cultured strongly influence exosome content. For instance, subjecting stem cells to mild stress (like low oxygen, or “hypoxia”) can upregulate the production of pro-angiogenic and osteogenic factors in their exosomes. This method mimics how the body naturally adapts to injury.Genetic Modification of Donor Cells
Scientists can insert specific genes into parent cells to overexpress therapeutic molecules—such as miRNAs that promote cartilage regeneration or growth factors that induce bone formation. These engineered cells then secrete exosomes enriched with beneficial signals.Direct Loading and Surface Engineering
After isolation, exosomes can be loaded with drugs, nucleic acids, or peptides using techniques like electroporation or chemical conjugation. Surface modification can also attach targeting ligands that guide exosomes to injured tissues, increasing precision.Scalable Production and Purification
Producing large quantities of high-quality exosomes is critical for clinical translation. Advances such as bioreactor-based cell cultures and microfluidic purification systems are enabling reproducible manufacturing while preserving biological function.
Together, these engineering approaches allow scientists to design exosomes that are stronger, smarter, and more specific for musculoskeletal repair.
Smart Delivery Systems: Getting Exosomes to Where They Are Needed
The next challenge lies in delivering these engineered exosomes effectively. When injected directly into the bloodstream, exosomes are rapidly cleared by organs like the liver and spleen. To overcome this, researchers are integrating exosomes with biomaterial-based delivery systems.
Biomaterial-Assisted Delivery Strategies
Hydrogels:
These water-rich polymer networks can encapsulate exosomes and release them gradually at the injury site. For instance, an injectable hydrogel loaded with bone marrow stem cell-derived exosomes can promote sustained bone regeneration.Scaffolds and Patches:
For bone and cartilage defects, 3D-printed or natural scaffolds made of collagen, chitosan, or biodegradable polymers can both support tissue growth and act as exosome carriers.Stimuli-Responsive Systems:
“Smart” biomaterials can release exosomes in response to specific triggers such as pH change, enzyme activity, or mechanical stress—ensuring that exosomes act only when and where they are needed.Local vs. Systemic Administration:
Localized delivery, such as direct injection into a joint or bone defect, provides higher therapeutic concentration and reduces unwanted distribution elsewhere in the body.
By combining nanotechnology and biomaterial engineering, researchers are ensuring that exosomes not only reach the target site but also stay active long enough to stimulate regeneration.
Applications in Musculoskeletal Regeneration
The potential of exosome therapy spans multiple areas of musculoskeletal medicine:
Bone Repair and Osteogenesis:
Exosomes derived from mesenchymal stem cells (MSCs) can enhance the differentiation of osteoblasts, promote angiogenesis (blood vessel formation), and suppress osteoclasts that break down bone tissue. When embedded in bone scaffolds, they accelerate healing of fractures and bone defects.Cartilage Regeneration and Osteoarthritis:
Cartilage has limited self-healing ability due to poor blood supply. Exosomes can modulate inflammatory pathways, protect chondrocytes, and stimulate the synthesis of cartilage matrix proteins—offering a novel, cell-free approach for treating arthritis.Tendon and Muscle Healing:
In tendons and skeletal muscles, exosomes help regulate inflammation, recruit repair cells, and restore structural integrity. They can reduce scar formation and improve mechanical strength after injury.Joint and Systemic Crosstalk:
Interestingly, exosomes also facilitate communication between bone, muscle, and adipose tissues—suggesting they could restore balance in metabolic and age-related musculoskeletal disorders.
These applications show how exosomes bridge the gap between molecular signaling and tissue-level repair.
Current Challenges and Future Prospects
Despite impressive progress, several key challenges remain before exosome therapies can become routine clinical tools:
Standardization and Quality Control – Exosomes vary depending on cell type, culture conditions, and isolation methods. A universal standard for characterization and potency testing is essential.
Safety and Off-Target Risks – Since exosomes carry multiple bioactive molecules, their long-term effects must be thoroughly evaluated to avoid unintended immune or metabolic responses.
Scaling Up Manufacturing – Producing clinical-grade exosomes in large volumes without compromising function remains technically demanding.
Regulatory Hurdles – Clear frameworks for approval, dosage, and quality assurance are still evolving.
Nevertheless, advances in synthetic biology, biofabrication, and “designer exosomes” promise to overcome many of these barriers. Collaborative efforts between material scientists, clinicians, and molecular biologists will accelerate translation from laboratory to patient care.
Conclusion: A Glimpse into the Future of Regenerative Medicine
Exosome-based therapy represents a paradigm shift in how we approach tissue healing. Rather than replacing damaged tissue with artificial implants, we are now learning to reprogram the body’s own communication systems for repair. By engineering exosomes and integrating them with smart biomaterials, it may soon be possible to regenerate bones, muscles, and cartilage with unprecedented precision.
For students of biotechnology and biomedical sciences, exosome research offers a powerful intersection of cell biology, nanotechnology, and biomaterial science—a true reflection of modern interdisciplinary medicine. As research continues, these tiny messengers may well redefine how we heal from within.
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en