Disaccharides and Polysaccharides: Structure, Examples & Functions
Understand disaccharides and polysaccharides—structure, glycosidic bonds, examples, and biological roles. A clear guide for undergraduate biology students.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
10/7/20257 min read
Carbohydrates are among the most essential biomolecules in living organisms. They consist mainly of carbon (C), hydrogen (H), and oxygen (O), generally in the ratio (CH₂O)n. Carbohydrates act as the primary source of energy, form structural components of cells, and function as precursors for nucleotides and lipids.
They are broadly classified into four types based on the number of sugar units:
Monosaccharides – Simple sugars (e.g., glucose, fructose, galactose)
Disaccharides – Two monosaccharides linked together
Oligosaccharides – 3–10 monosaccharide units
Polysaccharides – Long chains of monosaccharides, often in hundreds or thousands
This note focuses on disaccharides and polysaccharides, emphasizing their structure, formation, and biological significance. To understand them, it is essential first to know about the glycosidic bond, which links sugar units together.
Glycosidic Bond – The Link Between Sugar Units
Definition:
A glycosidic bond is a covalent linkage formed between two monosaccharide molecules (or between a sugar and another non-sugar group). It is the fundamental connection that holds carbohydrate chains together.
Formation of Glycosidic Bond
A glycosidic bond forms through a condensation (dehydration) reaction, where a molecule of water (H₂O) is removed. The hydroxyl group (-OH) from the anomeric carbon of one monosaccharide reacts with the hydroxyl group of another monosaccharide, resulting in the formation of a C–O–C bond.
Example:
Glucose + Glucose → Maltose + H₂O
Here, an α(1→4) glycosidic bond is formed between the first carbon of one glucose molecule and the fourth carbon of the second.
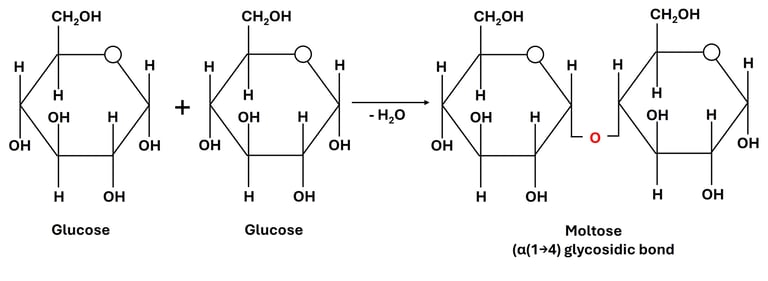
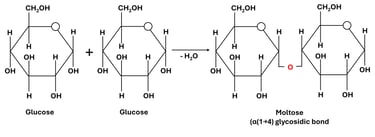
Fig 1: Formation of Glycosidic Bonds
Types of Glycosidic Bonds
1. Based on Carbon Position:
(1→4) linkage: between carbon 1 of one sugar and carbon 4 of another (e.g., maltose, amylose).
(1→6) linkage: between carbon 1 and carbon 6 (e.g., branching points in glycogen and amylopectin).
(1→2) linkage: between carbon 1 of one sugar and carbon 2 of another (e.g., sucrose).
2. Based on Orientation:
α (alpha) linkage: formed when the –OH on the anomeric carbon is below the plane of the ring (e.g., starch, glycogen).
β (beta) linkage: formed when the –OH is above the plane (e.g., cellulose, chitin).
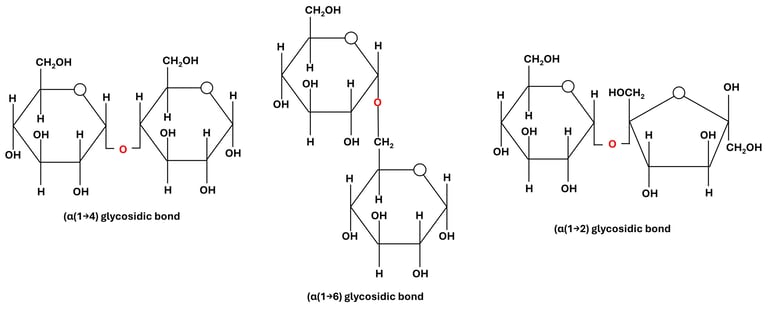
Fig 2: Glycosidic Bonds Based on Carbon Position
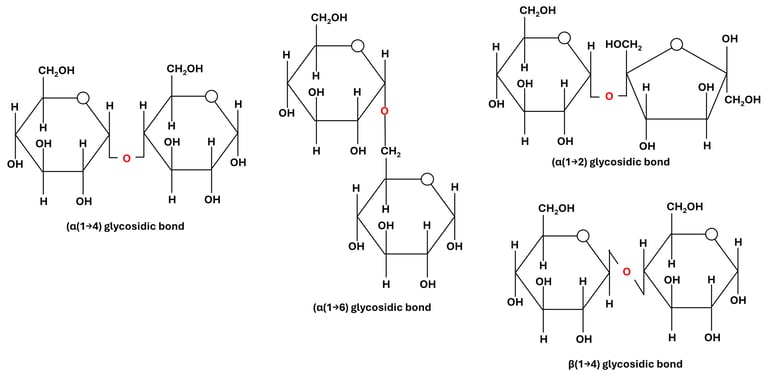
Fig 3: Glycosidic Bonds Based on Orientation
Biological Importance of Glycosidic Bonds
Determine Structure: The linkage type dictates whether a carbohydrate is helical (starch) or fibrous (cellulose).
Affect Digestibility: Enzymes are specific to bond types; humans can hydrolyze α-bonds (starch) but not β-bonds (cellulose).
Energy Release: Hydrolysis of glycosidic bonds releases monosaccharides used for energy.
Cellular Functions: Glycosidic linkages also attach sugars to proteins (glycoproteins) and lipids (glycolipids) for cell recognition and signaling.
Disaccharides
Definition:
Disaccharides are carbohydrates composed of two monosaccharide units that are chemically bonded together. These units are linked through a glycosidic bond, which forms via a condensation reaction — a process in which a molecule of water (H₂O) is released when the two sugar molecules join.
General Formula:
Most disaccharides follow the general chemical formula C₁₂H₂₂O₁₁, which reflects the loss of one water molecule when two hexose sugars (C₆H₁₂O₆ each) combine.
Formation:
The formation of a disaccharide occurs when the hydroxyl group (-OH) of one monosaccharide reacts with the hydroxyl group of another, typically involving the anomeric carbon (the carbon derived from the carbonyl group in the open-chain form) of one sugar molecule (Fig 1).
During this reaction:
The two hydroxyl groups interact.
A molecule of water (H₂O) is released (condensation).
A glycosidic bond (C–O–C linkage) is formed, connecting the two sugar units.
Depending on which carbons participate in the bond and their orientation (α or β form), different types of glycosidic bonds can form — such as α(1→4), β(1→4), or α(1→2) linkages (Fig 2 and Fig 3).
Examples:
Sucrose (Cane sugar): Formed from glucose + fructose via an α(1→2) bond.
Maltose: Formed from two glucose molecules linked by an α(1→4) bond.
Lactose (Milk sugar): Formed from galactose + glucose via a β(1→4) bond.
Each of these disaccharides has unique properties — for example, sucrose is non-reducing, while maltose and lactose are reducing sugars because they have a free anomeric carbon capable of acting as a reducing agent.
Important Disaccharides
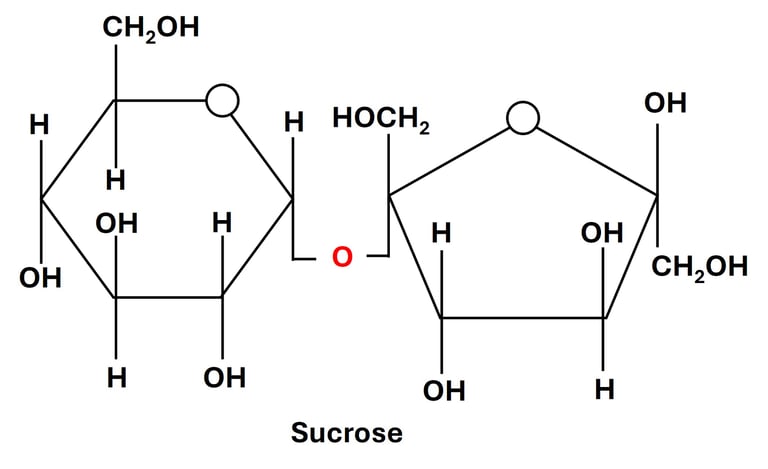
1. Sucrose (Glucose + Fructose)
Linkage: α(1→2) glycosidic bond between the anomeric carbon (C1) of glucose and the anomeric carbon (C2) of fructose.
Source: Found abundantly in sugarcane, sugar beet, and many fruits such as pineapple and mango.
Property: Non-reducing sugar — because both anomeric carbons are involved in the glycosidic bond, there is no free aldehyde or keto group to act as a reducing agent.
Function:
Acts as the primary transport form of carbohydrate in plants, moving through the phloem to supply energy to various tissues.
In humans and animals, sucrose serves as a major dietary source of energy, rapidly metabolized into glucose and fructose during digestion.
Enzyme action: The enzyme sucrase (invertase) hydrolyzes sucrose into its component monosaccharides, glucose and fructose.
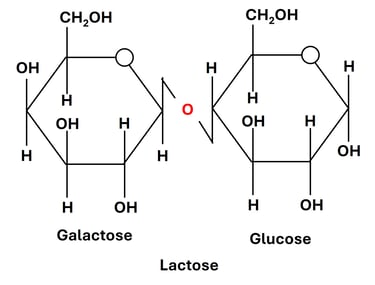
2. Lactose (Galactose + Glucose)
Linkage: β(1→4) glycosidic bond between the anomeric carbon (C1) of galactose and the hydroxyl group on the C4 of glucose.
Source: Found naturally in milk and dairy products, hence commonly known as milk sugar.
Property: Reducing sugar — since the glucose unit retains a free anomeric carbon, lactose can participate in redox reactions such as Benedict’s or Fehling’s tests.
Function:
Serves as an important energy source for infants and young mammals.
Plays a role in calcium absorption and gut microbiota development.
Enzyme: The enzyme lactase, produced in the small intestine, catalyzes the hydrolysis of lactose into glucose and galactose.
Note: Lactase deficiency leads to lactose intolerance, a condition in which undigested lactose causes bloating, gas, and diarrhea due to fermentation by intestinal bacteria.

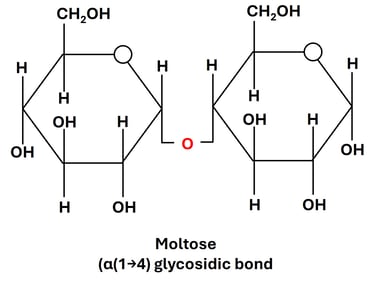
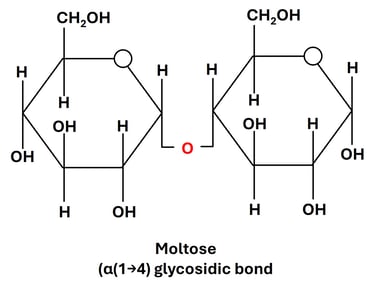
3. Maltose (Glucose + Glucose)
Linkage: α(1→4) glycosidic bond between the anomeric carbon (C1) of one glucose and the hydroxyl group on C4 of the second glucose.
Source: Formed during the partial hydrolysis of starch or glycogen, especially in germinating grains like barley, and during starch digestion in humans.
Property: Reducing sugar, as one of the glucose units has a free anomeric carbon.
Function:
Acts as an intermediate product in the digestion of polysaccharides like starch and glycogen.
Provides a quick source of glucose when broken down by the enzyme maltase in the small intestine.
Found in malted foods and beverages, giving them a characteristic sweet flavor.
Polysaccharides
Definition:
Polysaccharides are complex carbohydrates formed by the polymerization of numerous monosaccharide units (generally glucose or its derivatives) joined through glycosidic bonds. Because of their large size and structural complexity, they are often referred to as macromolecular carbohydrates or glycans. These molecules may consist of hundreds to thousands of sugar units, giving them diverse physical and biological properties.
Structural Characteristics
Linear Polysaccharides:
These have a straight, unbranched chain of monosaccharide residues connected end-to-end.
Example: Cellulose and amylose.
Their uniform, linear arrangement allows close packing of chains, resulting in fibrous, rigid structures — ideal for providing mechanical strength and structural support in plants.
Branched Polysaccharides:
These consist of a main chain with side chains (branches) of monosaccharides attached at various points.
Example: Glycogen and amylopectin.
The branching provides multiple ends for enzymatic action, making these molecules efficient for rapid energy release and storage.
Classification of Polysaccharides
Polysaccharides are broadly classified into two major types based on their biological functions:
1. Storage Polysaccharides
These serve as energy reserves in living organisms. When energy is required, enzymes can quickly hydrolyze them into glucose molecules.
Starch (in plants):
Composed of two components — amylose (linear α-1,4 linked glucose units) and amylopectin (branched with α-1,6 linkages).
Stored mainly in plant seeds and tubers such as rice, wheat, and potatoes.
Serves as the primary energy storage molecule in plants.
Glycogen (in animals and fungi):
Structurally similar to amylopectin but more highly branched.
Found in liver and muscle tissues in animals.
Allows rapid mobilization of glucose when energy demand increases — for example, during exercise or fasting.
2. Structural Polysaccharides
These provide mechanical strength, rigidity, and protection to cells and tissues. They are usually insoluble and resistant to enzymatic digestion.
Cellulose (in plants):
Composed of β(1→4) linked glucose units forming long, unbranched chains.
These chains align parallel to form microfibrils, giving plant cell walls tensile strength.
Humans cannot digest cellulose due to the absence of the enzyme cellulase, though it serves as dietary fiber aiding digestion.
Chitin (in arthropods and fungi):
Structurally similar to cellulose but made of N-acetylglucosamine units linked by β(1→4) bonds.
Forms the exoskeleton of insects, crabs, and lobsters, and also strengthens fungal cell walls.
Comparison Between Disaccharides and Polysaccharides
FAQs
1. What is a glycosidic bond?
It is a covalent bond formed between the anomeric carbon of one monosaccharide and a hydroxyl group of another, linking sugar units together.
2. Why can’t humans digest cellulose?
Humans lack the enzyme cellulase, which breaks β(1→4) bonds present in cellulose.
3. What makes sucrose non-reducing?
Both anomeric carbons of glucose and fructose are involved in the glycosidic bond, leaving no free reducing group.
4. Why is glycogen more efficient for energy storage than starch?
Because of its highly branched structure, glycogen can be rapidly hydrolyzed to release glucose when needed.
5. What is the biological significance of α and β linkages?
α-linkages (as in starch) are digestible and serve for energy storage, whereas β-linkages (as in cellulose) create rigid structures and are not digestible by humans.
References and Suggested Reading:
Nelson, D. L., & Cox, M. M. Lehninger Principles of Biochemistry, 9th Ed.
Voet, D., & Voet, J. G. Biochemistry, 5th Ed.
Berg, Tymoczko & Gatto. Biochemistry, 9th Ed.
Campbell, N. A., & Reece, J. B. Biology, Pearson Education.
Author Details
Dr. Mainak Mukhopadhyay
Associate Professor
Department of Biosciences
JIS University, Kolkata
(Ph.D. from Indian Institute of Technology Kharagpur, 2014)
Google Scholar Profile: https://scholar.google.com/citations?user=7mKAs4UAAAAJ&hl=en