Carbohydrate Stereochemistry: A Complete Guide for Students
Carbohydrate stereochemistry explained—D/L forms, cyclic structures, mutarotation, and their biological significance simplified.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
9/4/20257 min read
Introduction
Carbohydrates, often referred to as saccharides, are among the most abundant biomolecules in nature. They are not only the primary source of energy for living organisms but also play diverse roles in structural support, signaling, cell recognition, and immunity. Beyond their chemical composition of carbon, hydrogen, and oxygen, what makes carbohydrates truly fascinating is the three-dimensional arrangement of their atoms, also known as stereochemistry.
Stereochemistry deals with the spatial orientation of atoms in a molecule, and in the case of carbohydrates, it becomes particularly significant because most sugars contain multiple chiral centers. A chiral carbon atom, bonded to four different groups, gives rise to stereoisomers—molecules that share the same molecular formula but differ in their 3D arrangement. This subtle variation can dramatically influence biological activity. For instance, while D-glucose is the major energy currency in metabolism, its mirror image L-glucose is rarely metabolized by human enzymes.
Understanding carbohydrate stereochemistry is crucial because:
Biological specificity depends on stereochemistry. Enzymes and receptors can distinguish between isomers and will interact with only one particular configuration.
Medical and pharmaceutical applications often involve sugars or sugar mimics, where the orientation of hydroxyl groups determines drug activity.
Structural carbohydrates like cellulose and starch differ only in stereochemistry at one position, yet this difference makes cellulose indigestible for humans while starch is a staple food.
Thus, carbohydrate stereochemistry bridges organic chemistry, biochemistry, and biology, offering insights into how minute structural variations define the behavior of sugars in living systems.
Stereochemistry: Definition
Stereochemistry is the branch of chemistry that deals with the three-dimensional arrangement of atoms in molecules and the effect of this spatial arrangement on their physical and chemical properties. In simple terms, it studies how the same set of atoms can be connected in the same way but oriented differently in space, leading to molecules with distinct behaviors.
In the context of carbohydrates, stereochemistry is especially important because:
Most sugars contain multiple chiral centers (carbons attached to four different groups).
The presence of these chiral centers gives rise to isomers that have the same molecular formula but differ in their 3D arrangement.
These differences directly affect biological activity, enzyme recognition, and metabolism.
📌 Example:
D-glucose and L-glucose are stereoisomers (mirror images). While D-glucose is readily used in cellular respiration, L-glucose is not metabolized by human enzymes.
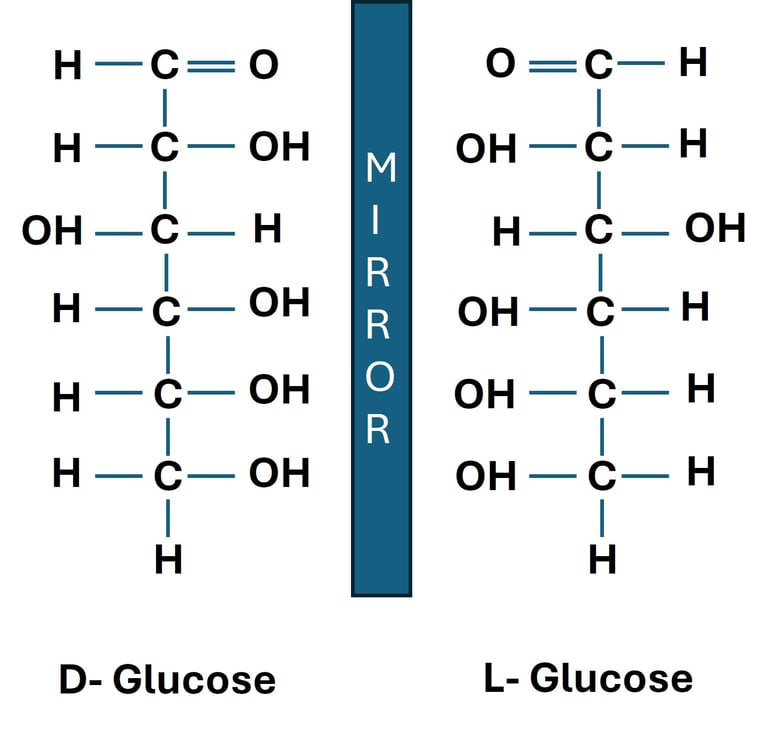
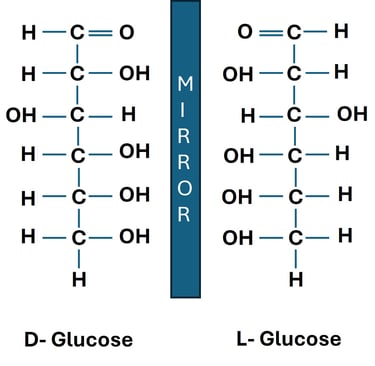
Chirality in Carbohydrates
One of the most fundamental concepts in carbohydrate stereochemistry is chirality. A molecule is said to be chiral if it cannot be superimposed on its mirror image, much like how our left and right hands are mirror images but not identical.
Chiral Carbon (Asymmetric Carbon)
A chiral carbon atom is a carbon bonded to four different atoms or groups. The different possible spatial arrangements of these groups lead to stereoisomers.
In carbohydrates:
Most monosaccharides contain multiple chiral carbons, which increases the number of possible stereoisomers.
The general rule for the maximum number of stereoisomers:
Number of stereoisomers=2n\text{Number of stereoisomers} = 2^nNumber of stereoisomers=2n
where n = number of chiral carbons.
📌 Example:
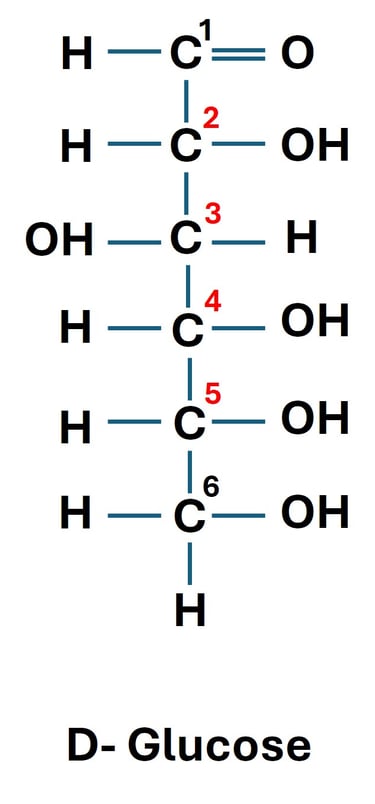
Glucose (an aldohexose) has 4 chiral carbons (C-2, C-3, C-4, C-5).
Possible stereoisomers = 24=162^4 = 1624=16.
These 16 stereoisomers include D- and L-glucose as well as other related sugars like mannose and galactose.
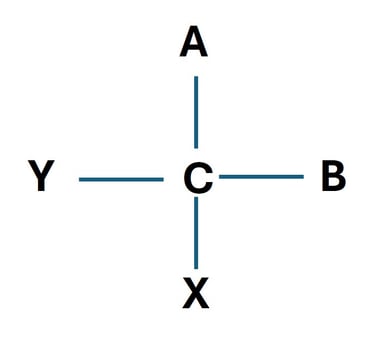
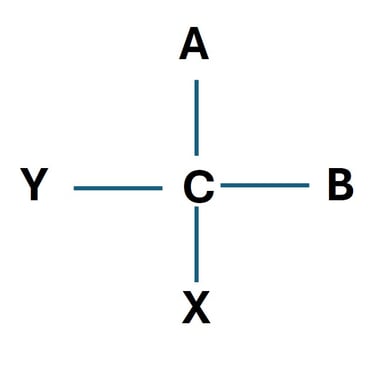
D- and L- Configuration in Carbohydrates
The stereochemistry of monosaccharides is commonly described using the D- and L-system, which is based on the configuration of the simplest sugar, glyceraldehyde. This system does not depend on optical rotation (+ or –), but rather on the position of the hydroxyl group (-OH) on the penultimate carbon atom in a Fischer projection.
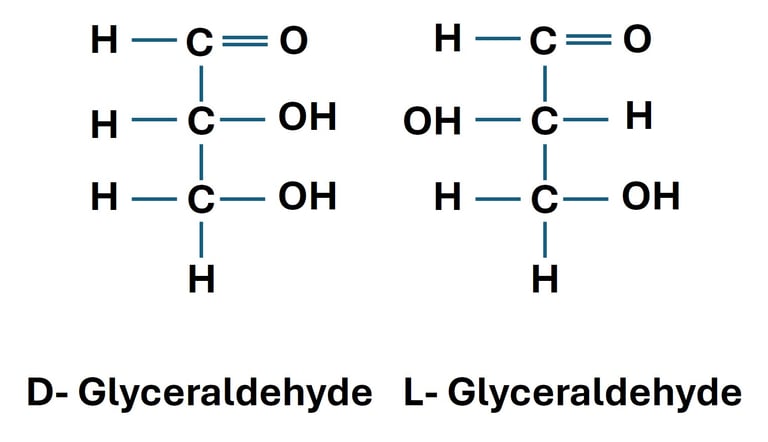
The Glyceraldehyde Reference
Glyceraldehyde is the simplest aldose (three-carbon sugar) with one chiral carbon.
Two stereoisomers exist:
D-glyceraldehyde: –OH group on the right side of the chiral carbon in Fischer projection.
L-glyceraldehyde: –OH group on the left side.
All monosaccharides are classified as D- or L- by comparing their structure to glyceraldehyde.
The Penultimate Carbon Rule
In Fischer projections of monosaccharides, the penultimate carbon (the chiral carbon farthest from the carbonyl group) determines the configuration.
If the –OH group on this carbon is:
Right side → D-sugar
Left side → L-sugar
📌 Example:
D-glucose → –OH at C-5 is on the right.
L-glucose → –OH at C-5 is on the left.
Important Clarification: D/L ≠ +/–
The D- and L- system refers to configuration (structural orientation relative to glyceraldehyde).
The optical activity (+ or –) refers to how the molecule rotates plane-polarized light.
A D-sugar can be dextrorotatory (+) or levorotatory (–).
Example: D-glucose is (+), but D-fructose is (–).
Prevalence in Nature
Most naturally occurring sugars (glucose, galactose, mannose, ribose, etc.) belong to the D-series.
L-sugars are relatively rare in nature but are found in certain glycoproteins and bacterial cell walls.
Biological Significance
Enzymes are highly stereospecific and generally recognize only D-sugars.
This stereospecificity is why D-glucose serves as the universal energy source, while L-glucose is not metabolized in humans.
In pharmaceuticals, D- and L-sugars are used differently because even a small change in configuration alters drug-receptor interactions.
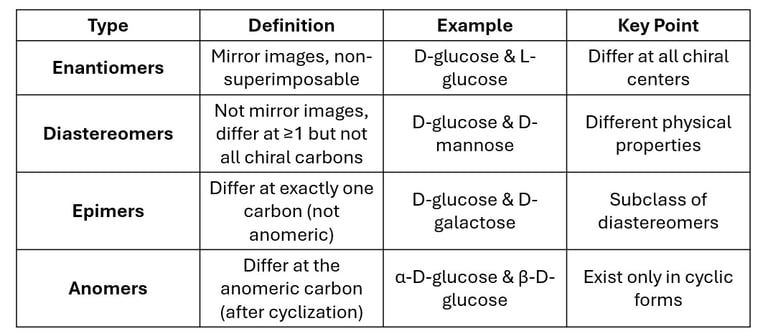
1. Enantiomers
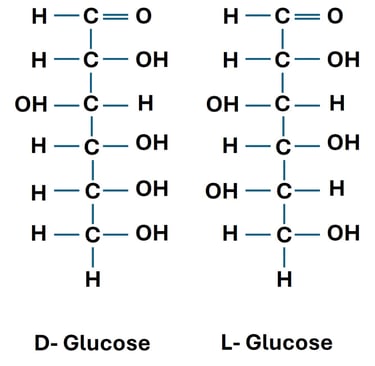
Definition: Enantiomers are pairs of molecules that are non-superimposable mirror images of each other.
They have the same physical properties (melting point, solubility, etc.) except for their optical rotation and behavior in chiral environments.
Example:
D-glucose and L-glucose are enantiomers.
Both have the same molecular formula (C₆H₁₂O₆), but their hydroxyl groups are arranged in mirror-image fashion.
Biological Relevance: Human enzymes recognize D-glucose, but not L-glucose, showing the importance of stereochemistry in metabolism.
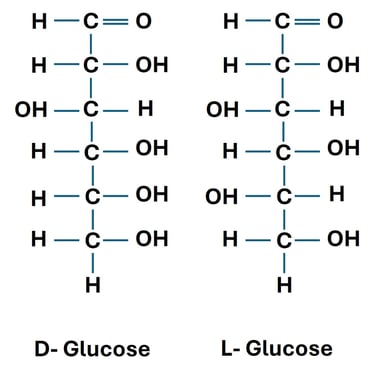
2. Diastereomers
Definition: Diastereomers are stereoisomers that are not mirror images of each other.
They differ at one or more (but not all) chiral centers.
Example:
D-glucose and D-mannose are diastereomers (different at C-2).
D-glucose and D-galactose are also diastereomers (different at C-4).
Biological Relevance: Although diastereomers share some properties, they may be metabolized differently by enzymes.
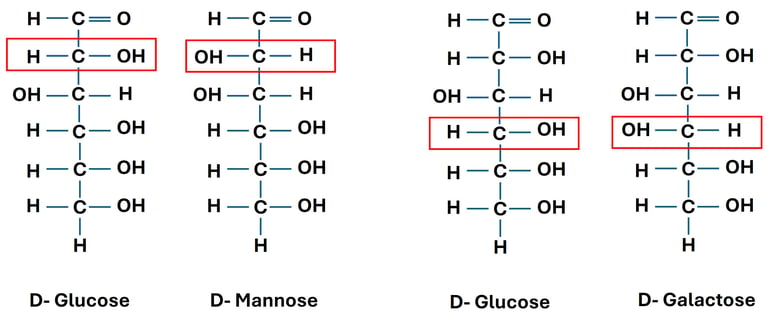
3. Epimers
Definition: Epimers are a special type of diastereomers that differ in configuration at only one specific carbon atom (except the anomeric carbon).
Examples:
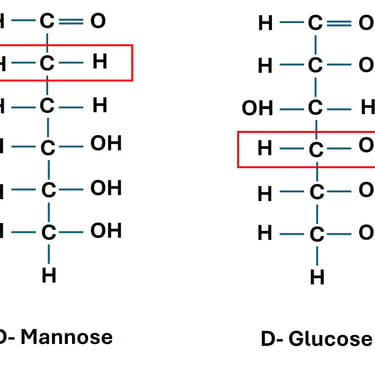
D-glucose and D-mannose → differ at C-2 → C-2 epimers.
D-glucose and D-galactose → differ at C-4 → C-4 epimers.
Biological Relevance: Epimers often have distinct metabolic pathways. For instance, epimerization (conversion between epimers) is catalyzed by specific enzymes like epimerases. (Fig same as above)
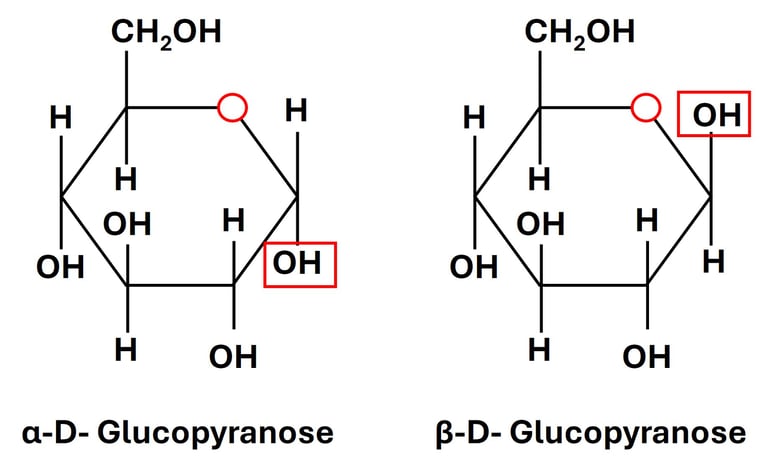
4. Anomers
Definition: Anomers are isomers that differ at the anomeric carbon (the carbon derived from the carbonyl group during ring formation).
When a sugar cyclizes into a ring, the carbonyl carbon (C-1 in aldoses, C-2 in ketoses) becomes a new chiral center, called the anomeric carbon.
Two possible forms:
α-anomer: –OH group on the anomeric carbon is on the opposite side of the ring from the CH₂OH group (down in Haworth projection for D-sugars).
β-anomer: –OH group on the anomeric carbon is on the same side as the CH₂OH group (up in Haworth projection for D-sugars).
Example:
α-D-glucose and β-D-glucose are anomers.
Biological Relevance: The α- and β-forms of glucose interconvert in solution (mutarotation), and this difference is vital in polysaccharide structures:
Starch contains α-linkages.
Cellulose contains β-linkages.
5. Summary Table
✅ Frequently Asked Questions (FAQs)
Q1. What is stereochemistry in carbohydrates?
Stereochemistry refers to the three-dimensional arrangement of atoms in carbohydrate molecules and how this arrangement influences their properties and biological functions.
Q2. What is a chiral carbon?
A carbon atom bonded to four different groups is called a chiral (asymmetric) carbon.
Q3. Why is chirality important in carbohydrates?
Because enzymes and receptors are stereospecific, meaning they can distinguish between different stereoisomers of sugars.
Q4. What is the difference between D- and L-sugars?
They are based on the orientation of the hydroxyl group on the penultimate carbon compared to glyceraldehyde.
Q5. Do D- and L-sugars correspond to optical rotation (+/–)?
No. D/L denotes configuration, while +/– denotes the direction of optical rotation.
Q6. Why are most naturally occurring sugars D-sugars?
It is an evolutionary selection. Enzymes evolved stereospecificity for D-sugars to optimize metabolism.
Q7. What are enantiomers in carbohydrates?
Enantiomers are mirror-image stereoisomers, such as D-glucose and L-glucose.
Q8. What are diastereomers?
Diastereomers are stereoisomers that are not mirror images, e.g., D-glucose and D-mannose.
Q9. What are epimers?
Epimers differ at only one chiral carbon (not the anomeric carbon), e.g., D-glucose and D-galactose.
Q10. What are anomers?
Anomers differ at the anomeric carbon formed during ring closure (α- and β-forms of glucose).
Q11. What is mutarotation?
It is the interconversion between α and β anomers in aqueous solution.
Q12. How many stereoisomers are possible for an aldohexose like glucose?
With 4 chiral centers, the number is 24=162^4 = 1624=16.
Q13. Why is cellulose indigestible for humans while starch is digestible?
Because cellulose has β(1→4) linkages, while starch has α(1→4) linkages; human enzymes cannot break β-linkages.
Q14. Can L-sugars be used in the food industry?
Yes, L-sugars like L-glucose are studied as low-calorie sweeteners because they are not metabolized by humans.
Q15. What role does stereochemistry play in drug design?
Many drugs mimic carbohydrate stereochemistry; a change in stereochemistry can make a drug inactive or harmful.
🧠 Critical Thinking Questions
Why do enzymes show high stereospecificity for D-sugars but not L-sugars?
If glucose has 16 stereoisomers, why do living organisms prefer only one (D-glucose)?
How might life be different if enzymes had evolved to use L-sugars instead of D-sugars?
Compare the metabolic consequences of consuming D-glucose vs. L-glucose.
Why do α- and β-anomers of glucose form different polysaccharides (starch vs. cellulose)?
How does the stereochemistry of sugars influence disease mechanisms such as viral infections?
If you changed just one hydroxyl group in glucose, how would it affect enzyme recognition?
Why might pharmaceutical companies synthesize L-sugars even though they are rare in nature?
Could organisms theoretically evolve to metabolize both D- and L-sugars? What would be the advantages or disadvantages?
How does carbohydrate stereochemistry illustrate the relationship between structure and biological function?
Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.