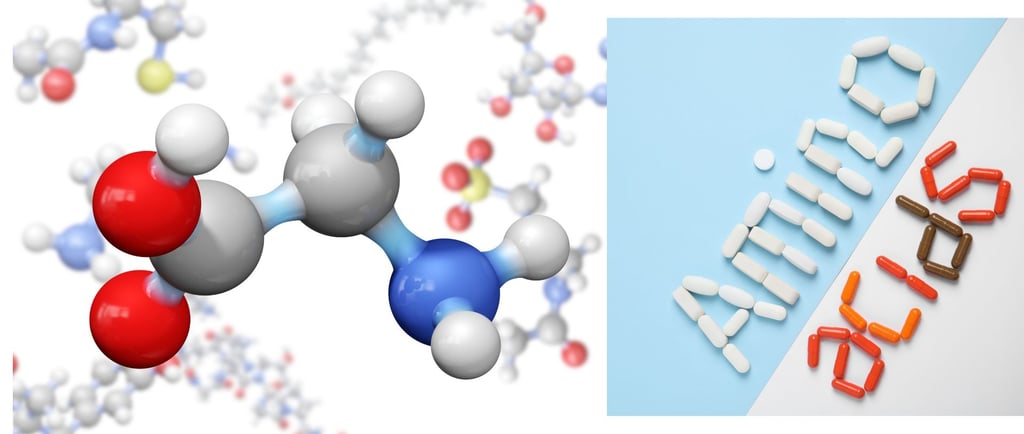
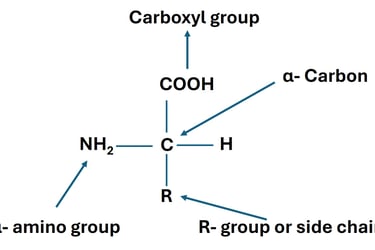
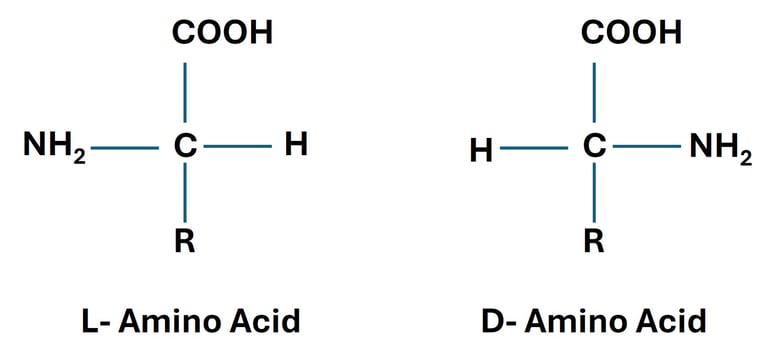
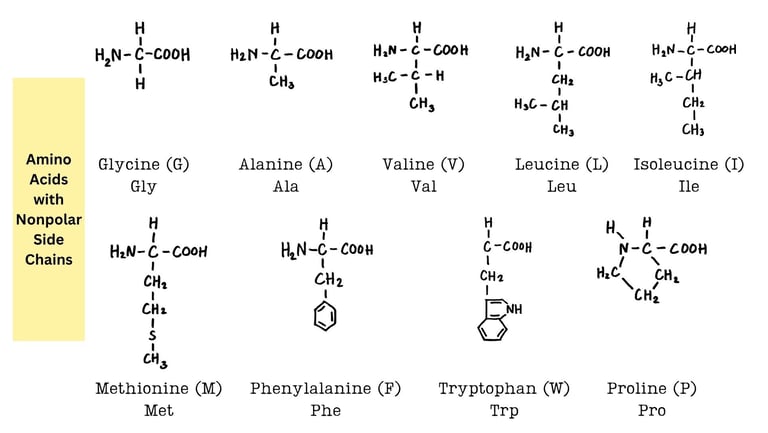
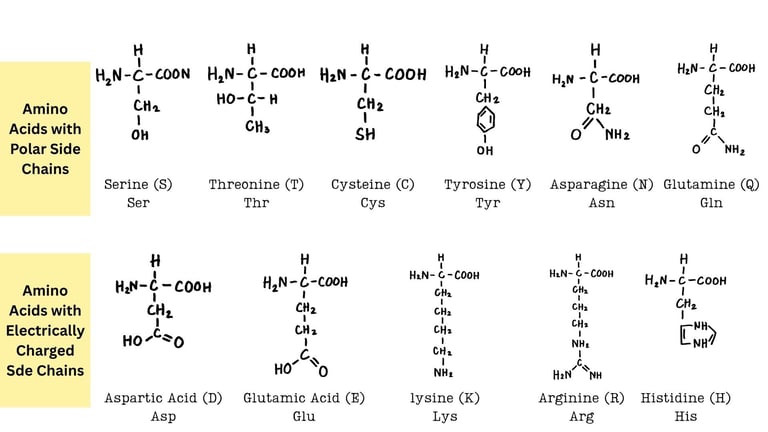
Amino Acids: Introduction, Classification, and Structure
Explore the chemistry of amino acids — their structure, types, ionization, and roles in protein synthesis. A comprehensive guide for undergraduate life science students.
BIOTECHNOLOGY
Dr. Mainak Mukhopadhyay
10/8/2025


Explore
Stay updated with biotech insights and research.
Connect
Discover
m.mukhopadhyay1212@gmail.com
+91-8777294577
© 2025. All rights reserved.